Genetic Diseases of Fucosylation: Insights from Model Organisms
- PMID: 40725456
- PMCID: PMC12295658
- DOI: 10.3390/genes16070800
Genetic Diseases of Fucosylation: Insights from Model Organisms
Abstract
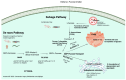
Fucosylation plays a fundamental role in maintaining cellular functions and biological processes across all animals. As a form of glycosylation, it involves the biochemical addition of fucose, a six-carbon monosaccharide, to biological molecules like lipids, proteins, and glycan chains. This modification is essential for optimizing cellular interactions required for receptor-ligand binding, cell adhesion, immune responses, and development. Disruptions in cellular fucose synthesis or in the mechanisms enabling its transfer to other molecules have been linked to human disease. Inherited defects in the fucosylation pathway are rare, with about thirty patients described. Through genome-wide association studies (GWAS), variants in fucosylation pathway genes have been associated with complex diseases such as glaucoma and stroke, and somatic mutations are often found in cancers. Recent studies have applied targeted genetic animal models to elucidate the mechanisms through which disruptions in fucosylation contribute to disease pathogenesis and progression. Key focus areas include GDP-fucose synthesis through de novo or salvage pathways, GDP-fucose transport into the Golgi and endoplasmic reticulum (ER), and its transfer by fucosyltransferases (FUTs) or protein O-fucosyltransferases (POFUTs) onto acceptor molecules. Loss or gain of function fucosylation gene mutations in animal models such as mice, zebrafish, and invertebrates have provided insights into some fucosylation disease pathogenesis. This review aims to bring together these findings, summarizing key insights from existing animal studies to possibly infer fucosylation disease mechanisms in humans.
Keywords: C. elegans; CDG IIC; LADII; drosophila; epilepsy; fucosylation; glaucoma; leukocytosis; mouse; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Skurska E., Szulc B., Kreczko K., Olczak M. Mutations in the SLC35C1 gene, contributing to significant differences in fucosylation patterns, may underlie the diverse phenotypic manifestations observed in leukocyte adhesion deficiency type II patients. Int. J. Biochem. Cell Biol. 2024;173:106602. doi: 10.1016/j.biocel.2024.106602. - DOI - PubMed
-
- Lu L., Varshney S., Yuan Y., Wei H.X., Tanwar A., Sundaram S., Nauman M., Haltiwanger R.S., Stanley P. In vivo evidence for GDP-fucose transport in the absence of transporter SLC35C1 and putative transporter SLC35C2. J. Biol. Chem. 2023;299:105406. doi: 10.1016/j.jbc.2023.105406. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

