Screening and Procedural Guidance for Mitral Transcatheter Edge-to-Edge Repair (M-TEER)
- PMID: 40725594
- PMCID: PMC12295293
- DOI: 10.3390/jcm14144902
Screening and Procedural Guidance for Mitral Transcatheter Edge-to-Edge Repair (M-TEER)
Abstract
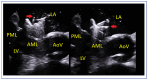
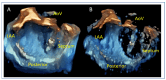
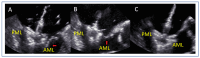
Mitral regurgitation (MR) is a common valvular heart disease associated with significant morbidity and mortality. For patients at high or prohibitive surgical risk, mitral transcatheter edge-to-edge repair (M-TEER) offers a less invasive alternative to surgery. This review outlines key aspects of patient selection and procedural planning for M-TEER, with a focus on clinical and echocardiographic criteria essential for success. Comprehensive imaging-especially 2D and 3D transesophageal echocardiography-is critical to assess leaflet anatomy, coaptation geometry, and mitral valve area. Selection criteria differ between primary and secondary MR and are guided by trials such as COAPT and MITRA-FR. Optimal outcomes rely on careful screening, anatomical suitability, and multidisciplinary evaluation. With growing experience and advancing technology, M-TEER has become a transformative option for treating severe MR in non-surgical candidates.
Keywords: MitraClip; TEER; mitral regurgitation; structural heart diseases; transcatheter edge-to-edge repair; transcatheter interventions; valvulopathies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
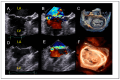
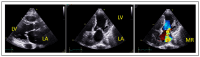
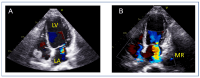
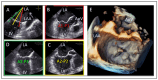

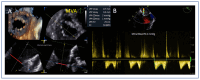


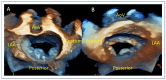
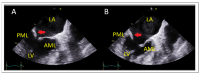
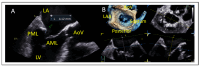
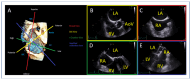
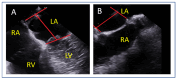
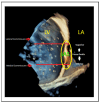

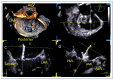
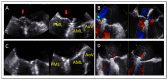
References
-
- Iung B., Baron G., Butchart E.G., Delahaye F., Gohlke-Bärwolf C., Levang O.W., Tornos P., Vanoverschelde J.-L., Vermeer F., Boersma E., et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003;24:1231–1243. doi: 10.1016/S0195-668X(03)00201-X. - DOI - PubMed
-
- Prakash R., Horsfall M., Markwick A., Pumar M., Lee L., Sinhal A., Joseph M.X., Chew D.P. Prognostic impact of moderate or severe mitral regurgitation (MR) irrespective of concomitant comorbidities: A retrospective matched cohort study. BMJ Open. 2014;4:e004984. doi: 10.1136/bmjopen-2014-004984. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

