Real-World Outcomes in FLT3-ITD Mutated Acute Myeloid Leukemia: Impact of NPM1 Mutations and Allogeneic Transplantation in a Retrospective Unicentric Cohort
- PMID: 40725802
- PMCID: PMC12294857
- DOI: 10.3390/jcm14145110
Real-World Outcomes in FLT3-ITD Mutated Acute Myeloid Leukemia: Impact of NPM1 Mutations and Allogeneic Transplantation in a Retrospective Unicentric Cohort
Abstract
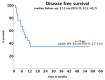
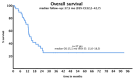
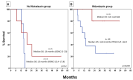
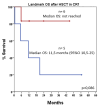
Background/Objectives: Acute myeloid leukemia (AML) with FLT3 internal tandem duplication (FLT3-ITD) mutations carries a poor prognosis. While FLT3 inhibitors like midostaurin show benefits in combination with chemotherapy, the role of allelic ratio (AR), NPM1 mutation status, and hematopoietic stem cell transplantation (HSCT) remains uncertain. Real-world data can help refine prognostic classification and treatment strategies. Methods: We retrospectively analyzed 37 fit patients with FLT3-ITD AML treated with standard "7+3" chemotherapy, with and without midostaurin, between 2013 and 2022. Patients were stratified by FLT3-ITD AR, NPM1 status, and treatment approach. Outcomes assessed included complete remission (CR), disease-free survival (DFS), and overall survival (OS). Results: Overall, 67.6% achieved CR/CRi. Response rates did not differ significantly by AR (low vs. high: 66.7% vs. 69.2%) or midostaurin use (72.6% vs. 60%; p = 0.49). NPM1 mutations were associated with improved DFS (10.3 vs. 3 months, p = 0.036) but not OS. HSCT, performed in 54.1% of patients, mainly in first remission (CR1), significantly prolonged DFS (not reached vs. 5.3 months, p = 0.005) and remained an independent predictor in multivariate analysis (HR: 0.160, p = 0.039). OS (median 15.1 months) did not vary significantly across subgroups. Among patients achieving CR1, OS was significantly longer in those who underwent HSCT after midostaurin-based induction compared to those not transplanted (median OS not reached vs. 12.8 months; 95% CI, 6.9-18.7; p = 0.045), whereas no significant benefit was observed after standard induction. In a landmark analysis restricted to patients transplanted in CR1, those who had received midostaurin-based induction showed a trend toward improved OS compared to those treated with standard induction (median OS not reached vs. 11.5 months; 95% CI, 0.5-25.0; p = 0.086). Conclusions: This real-life study supports the importance of NPM1 mutations and HSCT in CR1, especially in the midostaurin era, for improving DFS in FLT3-ITD AML. These findings support updated guidelines for reducing the prognostic weight of AR and highlight the need for improved post-remission strategies in this setting.
Keywords: ELN guidelines; FLT3-ITD; acute myeloid leukemia; allelic ratio; midostaurin; overall survival.
Conflict of interest statement
CV was on the advisory board for Abbvie, Astellas, Jazz, BMS, and Otsuka. The remaining authors declare no relevant conflicts of interest.
Figures
References
-
- Oran B., Cortes J., Beitinjaneh A., Chen H.C., de Lima M., Patel K., Ravandi F., Wang X., Brandt M., Andersson B.S., et al. Allogeneic Transplantation in First Remission Improves Outcomes Irrespective of FLT3-ITD Allelic Ratio in FLT3-ITD–Positive Acute Myelogenous Leukemia. Biol. Blood Marrow Transplant. 2016;22:1218–1226. doi: 10.1016/j.bbmt.2016.03.027. - DOI - PMC - PubMed
-
- de Jonge H.J.M., Valk P.J.M., de Bont E.S.J.M., Schuringa J.J., Ossenkoppele G., Vellenga E., Huls G. Prognostic Impact of White Blood Cell Count in Intermediate Risk Acute Myeloid Leukemia: Relevance of Mutated NPM1 and FLT3-ITD. Haematologica. 2011;96:1310–1317. doi: 10.3324/haematol.2011.040592. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

