Immunosuppressants/Immunomodulators and Malignancy
- PMID: 40725854
- PMCID: PMC12295559
- DOI: 10.3390/jcm14145160
Immunosuppressants/Immunomodulators and Malignancy
Abstract
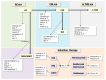
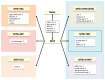
Individuals with immunosuppressive conditions are at a higher risk of developing malignancies than those in the general population. Immunosuppression weakens tumor immunity, hinders the detection of pro-oncogenic cells, and activates oncogenic viruses. Malignancies arising in immunosuppressed patients tend to be aggressive, have a high incidence of virus-associated cancers, and are reversible in some cases. Notably, immunosuppressive agents influence the frequency and type of malignancies, as well as their clinicopathological features. Organ transplant recipients receive long-term immunosuppressants to prevent acute rejection. Post-transplant malignancies vary depending on the type of drug administered before the onset of these diseases. Patients with rheumatoid arthritis (RA) are treated with long-term immunosuppressive medications, such as methotrexate (MTX). MTX is widely recognized as being associated with a specific type of lymphoproliferative disorder (LPD), known as MTX-associated LPD. Our recent report indicated that the clinicopathological features of rheumatoid arthritis-associated lymphoproliferative disorder (RA-LPD) also vary based on the other anti-RA agents used, such as tacrolimus and tumor necrosis factor inhibitors. Therefore, the clinicopathological characteristics of post-transplant LPD and RA-LPD evolve alongside the changes in the immunosuppressants/immunomodulators administered. Understanding the various characteristics and time trends of immunosuppressive neoplasms, particularly LPDs, in relation to different immunosuppressant/immunomodulator medications is highly valuable in clinical practice.
Keywords: anti-rheumatic agents; immunosuppressive agents; methotrexate; post-transplant lymphoproliferative disorders; post-transplant malignancy; rheumatoid arthritis-associated lymphoproliferative disorders; rheumatoid arthritis-associated malignancy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., Arber D.A., Hasserjian R.P., Le Beau M.M., et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. IARC; Lyon, France: 2017. pp. 443–464.
Publication types
LinkOut - more resources
Full Text Sources

