Distinct Roles for Thymic Stromal Lymphopoietin (TSLP) and IL-33 in Experimental Eosinophilic Esophagitis
- PMID: 40726298
- PMCID: PMC12590318
- DOI: 10.1111/all.16682
Distinct Roles for Thymic Stromal Lymphopoietin (TSLP) and IL-33 in Experimental Eosinophilic Esophagitis
Abstract
Rationale: Thymic stromal lymphopoietin (TSLP) and IL-33 are alarmins implicated in eosinophilic esophagitis (EoE) pathogenesis by activating multiple cells, including mast cells (MCs). Whether TSLP or IL-33 have a role in EoE and whether their activities are distinct requires further investigation.
Methods: Experimental EoE was induced in wild type (WT) Il33-/- and Crlf2-/- mice. TSLP or IL-5 were neutralized using antibodies. Esophageal histopathology was determined by H&E, anti-Ki67, anti-CD31, and anti-MBP staining. Esophageal RNA was subjected to RNA sequencing. Bone marrow-derived MCs were activated with TSLP and IL-13 was determined (ELISA).
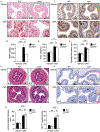
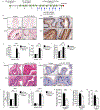
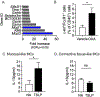
Results: TSLP and IL-33 were overexpressed in human and experimental EoE. Human and mouse esophageal MCs displayed the highest level of Crlf2 (TSLPR) compared to other immune cells. Crlf2-/- mice were nearly completely protected from EoE, and TSLP neutralization resulted in decreased basal cell proliferation, eosinophilia, lamina propria thickening, and vascularization. Induction of experimental EoE in Il33-/- mice resulted in reduced eosinophilia, but no alterations in tissue remodeling were observed compared to WT mice. RNA sequencing revealed that TSLP regulates the expression of key genes associated with human EoE (e.g., eotaxins, Il19, Klk5, Flg, Il36rn, Il1r2) and suggests a role for TSLP in regulating IL-1 signaling, barrier integrity, and epithelial cell differentiation. Experimental EoE was characterized by a MC-associated gene signature and elevated MCs. Activation of MCs with TSLP resulted in the secretion of IL-13.
Conclusion: TSLP and IL-33 have non-redundant functions in experimental EoE. This study highlights TSLP as an upstream regulator of IL-13 and a potential therapeutic target for EoE.
Keywords: IL‐33; TSLP; allergy; eosinophilic esophagitis.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Figures



Update of
-
Distinct roles for thymic stromal lymphopoietin (TSLP) and IL-33 in experimental eosinophilic esophagitis.bioRxiv [Preprint]. 2025 Mar 1:2025.02.25.640192. doi: 10.1101/2025.02.25.640192. bioRxiv. 2025. Update in: Allergy. 2025 Nov;80(11):3095-3107. doi: 10.1111/all.16682. PMID: 40060399 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- Israel Science Foundation
- US-Israel Bi-national Science Foundation
- AstraZeneca
- Israel Cancer Research Fund
- Israel Cancer Association USA
- Azrieli Foundation Canada-Israel
- Campaign Urging Research for Eosinophilic Disease
- P30 DK078392/DK/NIDDK NIH HHS/United States
- R37 AI045898/AI/NIAID NIH HHS/United States
- NH/NIH HHS/United States
- U19 AI070235/AI/NIAID NIH HHS/United States
- Sunshine Charitable Foundation
- R01 AI124355/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

