Intra-articular Injections of Allogeneic Platelet-Rich Plasma from Responder Patients for the Treatment of Knee Osteoarthritis: A Pilot and Feasibility Clinical Trial
- PMID: 40726366
- PMCID: PMC12307335
- DOI: 10.1177/19476035251355522
Intra-articular Injections of Allogeneic Platelet-Rich Plasma from Responder Patients for the Treatment of Knee Osteoarthritis: A Pilot and Feasibility Clinical Trial
Abstract
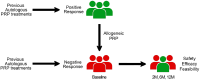
ObjectiveTo evaluate the feasibility, safety and efficacy of allogeneic platelet-rich plasma (PRP) from responder donors to treat knee osteoarthritis (KOA) patients who showed negative response to autologous PRP.DesignThis pilot feasibility trial included KOA patients who did not respond to previous autologous PRP treatment. They were treated with intra-articular injections of allogeneic PRP from responder donors. Patients filled out Knee injury and Osteoarthritis Outcome Score (KOOS), Visual Analogue Scale (VAS), and Lequesne Index at baseline, 2, 6, and 12 months. Blood and PRP from donors and patients were analyzed, and a cell proliferation study was carried out.ResultsOf the 16 patients enrolled, 14 completed the study. KOOS pain subscale and VAS showed a significant increase from baseline to 12 months, and the Lequesne Index to 6 months (P < .005). Six patients (42.9%) showed a Minimal Clinically Important Improvement. No adverse reactions to allogeneic PRP were reported. The platelet number between donors and recipients was similar (P > .05) with a platelet concentration factor of 2.5. Donors were significantly younger than patients (P < .05) and presented higher levels of IGF-1 (P < .05). Cell bioactivity showed no differences between patient and donor PRP (P > .05).ConclusionThe use of allogeneic PRP from donor responders is a feasible and safe treatment for KOA patients who do not respond to autologous PRP. This treatment showed efficacy after 1 year of follow-up, suggesting a valid alternative for these patients, although further research is needed.EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/). Registration number: 2021-001267-24.
Keywords: allogenic; growth factors; intra-articular injection; knee osteoarthritis; platelet-rich plasma.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Mahmoudian A, Lohmander LS, Mobasheri A, Englund M, Luyten FP. Early—stage symptomatic osteoarthritis of the knee—time for action. Nat Rev Rheumatol. 2021;17(10):621-32. - PubMed
-
- Livshetz I, Sussman BH, Papas V, Mohamed NS, Salem HS, Delanois RE, et al. Analyzing the burden of revision total knee arthroplasty in the United States between 2009 and 2016. J Knee Surg. 2023;36(2):121-31. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

