Transcriptomics combined with physiological analysis provides insights into the mechanism of resistance to Coleosporium bletiae in Bletilla striata
- PMID: 40726552
- PMCID: PMC12301390
- DOI: 10.3389/fpls.2025.1604512
Transcriptomics combined with physiological analysis provides insights into the mechanism of resistance to Coleosporium bletiae in Bletilla striata
Abstract
Introduction: Bletilla striata (Orchidaceae) is a valuable traditional Chinese medicinal plant prized for its dried rhizomes. However, its cultivation faces significant challenges from leaf rust disease caused by Coleosporium bletiae, which causes substantial yield losses.
Methods: To investigate host resistance mechanisms, we compared rust-resistant and susceptible B. striata accessions through integrated transcriptomic and physiological analyses.
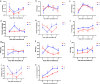
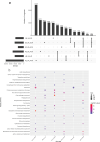
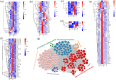
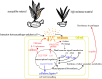
Results and discussion: Phenotypic observations revealed that while both resistant and susceptible plants developed rust spores by 2 days post-inoculation (dpi), the resistant accession exhibited a significantly slower progression of spore stack formation and lesion expansion on abaxial leaf surfaces over time. Integrated transcriptomic and physiological analyses revealed that the rust-resistant material of B. striata accessions exhibited faster and stronger defense responses to pathogen infection compared to susceptible plants. These responses were characterized by significant upregulation of DEGs associated with antioxidant defense systems, secondary metabolite biosynthesis, JA, SA, and BR signaling pathways, concurrent downregulation of DEGs involved in cell wall remodeling, and calcium-mediated signaling. Furthermore, rust pathogen inoculation triggered rapid physiological responses in resistant plants, including enhanced activity of defense-related enzymes (CAT, PAL, β-1,3-glucanase, and chitinase) and early accumulation of osmolytes (soluble sugars, soluble proteins, and proline). These coordinated molecular and biochemical responses effectively restricted pathogen colonization and spread. These findings delineate the molecular basis of rust resistance in B. striata, identifying key regulatory nodes in defense pathways that could be targeted through precision breeding or genetic engineering to develop durable resistance against C. bletilla.
Keywords: Bletilla striata; physiological analysis; resistant and susceptible material; rust pathogen; transcriptomics.
Copyright © 2025 Liu, Lu, Wu, Lu, Qin, Huang, Zou, Xia, Yang and Qiu.
Conflict of interest statement
Authors ZL and XZ were employed by the company Guilin Sanjin Pharmaceutical Co., Ltd. Authors RQ and KH were employed by the company Guangxi Yifang Tianjiang Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Anguelova-Merhar V. S., Westhuizen V. D., Pretorius Z. A. (2001). β-1,3-glucanase and chitinase activities and the resistance response of wheat to leaf rust. J. Phytopathol. doi: 10.1111/j.1439-0434.2001.tb03866.x - DOI
-
- Bates L. S., Waldren R. P., Teare I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1007/BF00018060 - DOI
-
- Baxter H. L., Stewart C. N. (2013). Effects of altered lignin biosynthesis on phenylpropanoid metabolism and plant stress. Biofuels 4, 635–650. doi: 10.4155/bfs.13.56 - DOI
-
- Benhamou N. (1991). Cell surface interactions between tomato and Clavibacter michiganense subsp. michiganense: localization of some polysaccharides and hydroxyproline-rich glycoproteins in infected host leaf tissues. Physiol. Mol. Plant Pathol. 38, 15–38. doi: 10.1016/S0885-5765(05)80140-7 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

