Management of aromatase inhibitor-associated bone loss (AIBL) in women with hormone-sensitive breast cancer: An updated joint position statement of the IOF, CABS, ECTS, IEG, ESCEO, IMS, and SIOG
- PMID: 40726588
- PMCID: PMC12301826
- DOI: 10.1016/j.jbo.2025.100694
Management of aromatase inhibitor-associated bone loss (AIBL) in women with hormone-sensitive breast cancer: An updated joint position statement of the IOF, CABS, ECTS, IEG, ESCEO, IMS, and SIOG
Abstract
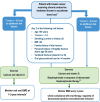
Background: Women with hormone-responsive breast cancer who receive adjuvant endocrine treatment with aromatase inhibitors (AI) are known to be at higher fracture risk due to a marked increase in bone resorption. In 2017, several interdisciplinary cancer and bone societies involved in the management of women with AI-associated bone loss (AIBL) published a joint position statement comprising evidence-based recommendations and a practical management algorithm for the assessment of fracture risk and optimal treatment of this patient population.
Patients and methods: In order to provide updated recommendations that reflect recent advances in the assessment and management of AIBL since publication of the 2017 joint position statement, a systematic literature review was undertaken to identify relevant studies for analysis, including systematic reviews and meta-analyses. Individual trials identified were assessed for their level of evidence based on design, size, follow-up, and evaluation of safety, as well as the impact of bone directed treatments on breast cancer outcomes.
Results: New evidence was combined with the existing recommendations to provide an updated joint position statement regarding fracture risk assessment and implementation of bone-directed therapy.
Conclusion: Current published literature, including recent clinical trial reports, systematic reviews and meta-analyses, continue to affirm the high risk of fractures in women with breast cancer who are receiving adjuvant AI treatment, a risk which has been observed to increase with the commonly used approach of extended duration AI therapy (>5 years). Risk factors for fracture and risk assessment in this patient population as well as the most suitable treatment modalities have been updated. Finally, the influence of bone protective treatments on breast cancer outcomes such as incidence of bone metastasis and breast cancer related overall survival have been included.
Keywords: Aromatase Inhibitor; Bisphosphonate; Breast cancer; Denosumab; Fracture; Osteoporosis.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Ginsburg O., Bray F., Coleman M.P., Vanderpuye V., Eniu A., Kotha S.R., Sarker M., Huong T.T., Allemani C., Dvaladze A., Gralow J., Yeates K., Taylor C., Oomman N., Krishnan S., Sullivan R., Kombe D., Blas M.M., Parham G., Kassami N., Conteh L. The global burden of women's cancers: a grand challenge in global health. Lancet. 2016 - PMC - PubMed
-
- Organization. 2024
-
- Caswell-Jin J.L., Sun L.P., Munoz D., Lu Y., Li Y., Huang H., Hampton J.M., Song J., Jayasekera J., Schechter C., Alagoz O., Stout N.K., Trentham-Dietz A., Lee S.J., Huang X., Mandelblatt J.S., Berry D.A., Kurian A.W., Plevritis S.K. Analysis of Breast Cancer Mortality in the US-1975 to 2019. JAMA. 2024;331(3):233–241. - PMC - PubMed
-
- Duffy S., Vulkan D., Cuckle H., Parmar D., Sheikh S., Smith R., Evans A., Blyuss O., Johns L., Ellis I., Sasieni P., Wale C., Myles J., Moss S. Annual mammographic screening to reduce breast cancer mortality in women from age 40 years: long-term follow-up of the UK Age RCT. Health Technol. Assess. 2020;24(55):1–24. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

