Assessing breakthrough infections: Covishield (late schedule) versus Covaxin - A prospective comparative analysis in the general population
- PMID: 40726698
- PMCID: PMC12296376
- DOI: 10.4103/jfmpc.jfmpc_1714_24
Assessing breakthrough infections: Covishield (late schedule) versus Covaxin - A prospective comparative analysis in the general population
Abstract
Introduction: Breakthrough infections (BTI) threaten the progress of the COVID-19 vaccination program towards pandemic control. To better understand the future vaccine, knowing the BTI in the general population over a while is important.
Aims and objectives: The study aimed to compare the BTI among the general population after 1 year of completion of the primary series of Covishield and Covaxin.
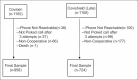
Materials and methods: This hospital-based prospective study was conducted among the general population beneficiaries of a COVID-19 vaccination center. Clients aged 18 years or above who had completed second vaccine dose were enrolled using systematic random sampling and followed up for 1 year. Using a semi-structured questionnaire, participants were telephonically interviewed within 1 month, 6 months, and 12 months of the second vaccine dose.
Results: Out of 1682 participants who completed the study, 958 (57.0%) and 724 (43.0%) participants received Covaxin and Covishield, respectively. Twenty-nine (3.0%) Covaxin recipients and 25 (3.5%) Covishield recipients reported BTI after 1 year of follow-up with no statistically significant difference among both groups (p-0.624). The binary logistic regression model showed that participants with either diabetes or hypertension had 1.22 times the risk of BTI compared to those without comorbidities (aOR: 1.219, CI: 0.072-20.716, p-0.891). BTI was not significantly associated with the type of vaccine, sex, employment status, and age category of the participants.
Conclusion: The study suggests that regardless of the type of vaccine received, the population will be at the same risk and require similar future containment strategies.
Keywords: BBV152 COVID-19 vaccine; Breakthrough Infections; COVID-19 Vaccines; ChAdOx1 nCoV-19; Pandemics; Vaccination.
Copyright: © 2025 Journal of Family Medicine and Primary Care.
Conflict of interest statement
There are no conflicts of interest.
Similar articles
-
Persistence of SARS-CoV-2 Antibodies for a Year Following SARS-CoV-2 Vaccinations (BBV152 and ChAdOx1 nCoV-19).Indian J Clin Biochem. 2025 Jan;40(1):111-120. doi: 10.1007/s12291-023-01149-w. Epub 2023 Sep 7. Indian J Clin Biochem. 2025. PMID: 39835239
-
COVID-19 Vaccines.2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 33355732 Free Books & Documents. Review.
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Vaccines for preventing infections in adults with haematological malignancies.Cochrane Database Syst Rev. 2025 May 21;5(5):CD015530. doi: 10.1002/14651858.CD015530.pub2. Cochrane Database Syst Rev. 2025. PMID: 40396505 Review.
References
-
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdO×1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–93. - PMC - PubMed
-
- Asokan MS, Joan RF, Babji S, Dayma G, Nadukkandy P, Subrahmanyam V, et al. Immunogenicity of SARS-CoV-2 vaccines BBV152 (COVAXIN®) and ChAdO×1 nCoV-19 (COVISHIELD™) in seronegative and seropositive individuals in India: A multicentre, nonrandomised observational study. Lancet Reg Health Southeast Asia. 2024;22:100361. doi: 10.1016/j.lansea.2024.100361. - PMC - PubMed
-
- Ministry of Health and Family Welfare. Gap between two doses of Covishield Vaccine extended from 6-8 weeks to 12-16 weeks based on recommendation of COVID Working Group. 2021. Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1718308 .
LinkOut - more resources
Full Text Sources