Prednisolone add-on in early phase schizophrenia: A randomized, double-blind, placebo-controlled pilot study
- PMID: 40726776
- PMCID: PMC12302662
- DOI: 10.1016/j.bbih.2025.101047
Prednisolone add-on in early phase schizophrenia: A randomized, double-blind, placebo-controlled pilot study
Abstract
Background: New pharmacological treatment strategies are urgently needed for schizophrenia since current antipsychotic medications have limitations related to efficacy, tolerability, and disease course modification. Different lines of evidence converge on aberrant activity of the immune system, but translation into new treatment is still pending. Studies investigating the antipsychotic properties of less potent anti-inflammatory drugs have provided equivocal findings. A potent agent like prednisolone might be better suited for establishing proof-of-concept that dampening of inflammation may be beneficial in schizophrenia.
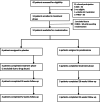
Methods: In this double-blind multicentre pilot study, 12 patients (aged 18-39) with schizophrenia maintained on stable antipsychotic regimens were randomized to prednisolone or placebo. Study medication was initiated with 40 mg/day and gradually tapered to zero over a period of 6 weeks. The primary outcome was difference in the Positive and Negative Syndrome Scale (PANSS) total score 6 weeks after baseline.
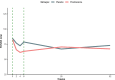
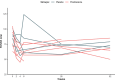
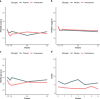
Outcomes: In patients randomized to prednisolone (N = 6), a clear trend towards more pronounced reduction of psychotic symptoms was observed compared to the placebo group (N = 6). Difference in symptom severity per the PANSS total score after 6 weeks of add-on treatment was 15·4 (SD = 8·5, p = 0·101). For the PANSS general score, we observed a statistically significant difference of 12·5 (SD = 4·6, p = 0·021) at week 6. Safety and tolerability were acceptable for the duration of prednisolone treatment.
Interpretation: These findings suggest beneficial effect of add-on prednisolone. However, this needs further replication in a larger sample for confirmation and to identify the mechanisms of action.
Keywords: Inflammation; PANSS general; PANSS total; Prednisolone; Psychosis; RCT; Schizophrenia; treatment.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Gunnhild Hoprekstad and Erik Johnsen reports financial support was provided by Western Norway Regional Health Authority. Iris E. C. Sommer reports a relationship with Gabather that includes: consulting or advisory. Iris E. C. Sommer reports a relationship with Otsuka that includes: consulting or advisory. Iris E. C. Sommer reports a relationship with 10.13039/501100013327Lundbeck that includes: funding grants. Iris E. C. Sommer reports a relationship with Janssen that includes: funding grants. Petros Drosos reports a relationship with Western Norway Regional Health Authority that includes: funding grants. Rune A. Kroken reports a relationship with Western Norway Regional Health Authority that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Addington D., Addington J., Maticka-Tyndale E., Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophrenia research. 1992;6(3):201–208. - PubMed
-
- Addington D., Addington J., Maticka-Tyndale E. Assessing depression in schizophrenia: the calgary depression scale. Br J Psychiatry Suppl. 1993;(22):39–44. - PubMed
-
- Balotsev R., Koido K., Vasar V., Janno S., Kriisa K., Mahlapuu R., et al. Inflammatory, cardio-metabolic and diabetic profiling of chronic schizophrenia. Eur Psychiatry. 2017;39:1–10. - PubMed
-
- Emsley R., Chiliza B., Asmal L. The evidence for illness progression after relapse in schizophrenia. Schizophr. Res. 2013;148(1–3):117–121. - PubMed
LinkOut - more resources
Full Text Sources

