Enhanced Neuroprotection by Diosgenin and Pterostilbene Combination Against Neurotoxicity Induced by Amyloid-Β 1-42 in SH-SY5Y Differentiated Cell Models
- PMID: 40726803
- PMCID: PMC12289621
- DOI: 10.1177/09727531251356049
Enhanced Neuroprotection by Diosgenin and Pterostilbene Combination Against Neurotoxicity Induced by Amyloid-Β 1-42 in SH-SY5Y Differentiated Cell Models
Abstract
Background: Alzheimer's disease (AD) is the predominant age-related neurodegenerative condition, characterised by the gradual and irreversible loss of neurons. Key pathological features include amyloid plaques and neurofibrillary tangles, which trigger a chronic inflammatory response in the brain, leading to microglial activation and proliferation.
Purpose: This study evaluated the neuroprotective potential of diosgenin (DGN) and pterostilbene, two phytoconstituents with known antioxidant and anti-inflammatory properties, in amyloid-β 1-42 exposed SH-SY5Y cells.
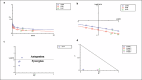
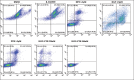
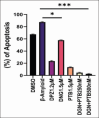
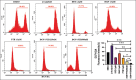
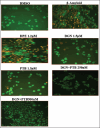
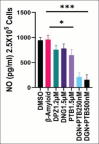
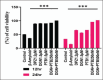
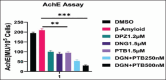
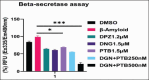
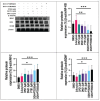
Methods: Human neuroblastoma cells were cultured and neurodifferentiated with retinoic acid, then exposed to amyloid-β 1-42 to simulate the AD model. Treatments included DGN (1.5 µM), pterostilbene (PTB) (1.5 µM), their combination (0.25 µM and 0.5 µM), and donepezil (1.2 µM) as a standard drug for comparison. The effects of treatments were assessed through cell viability, reactive oxygen species (ROS) levels, apoptosis, inflammatory cytokines, and BDNF levels using various assays, including flow cytometry, ELISA, Western blotting, and inhibitory assays for NOS, H2O2-mediated oxidative stress, DPPH, AChE, and β-secretase.
Results: DGN and PTB combination indicated increased cell viability, reduced microglial activation, decreased apoptosis, and lower ROS levels, with the maximum effect observed in the combination group (0.5 µM). Combination treatments also showed maximum inhibition in various assays and reduced levels of cytokines while upregulating BDNF, highlighting their neuroprotective, anti-inflammatory, and antioxidant activities.
Conclusion: The findings suggest that the combination of DGN and PTB may serve as an effective neuroinflammatory modulator in managing neurodegenerative diseases.
Keywords: Alzheimer’s disease; diosgenin; neuroinflammation; neuroprotection; pterostilbene.
© The Author(s) 2025.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Figures





Similar articles
-
Design, synthesis, and biological evaluation of caffeic acid-based novel multifunctional molecules for the management of Alzheimer's disease.Eur J Med Chem. 2025 Oct 15;296:117831. doi: 10.1016/j.ejmech.2025.117831. Epub 2025 Jun 10. Eur J Med Chem. 2025. PMID: 40578253
-
Evaluating the effectiveness of pterostilbene in improving memory and offering neuroprotection in a rat model of Alzheimer's disease induced by aluminum chloride.J Complement Integr Med. 2025 Jul 31. doi: 10.1515/jcim-2025-0142. Online ahead of print. J Complement Integr Med. 2025. PMID: 40740154
-
Investigation of neuroprotective effects of newly synthesized benzimidazolium salt against neurotoxicity in differentiated SH-SY5Y neuronal cells.Biochem Biophys Res Commun. 2025 Sep 1;777:152255. doi: 10.1016/j.bbrc.2025.152255. Epub 2025 Jun 25. Biochem Biophys Res Commun. 2025. PMID: 40580722
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer's disease.Health Technol Assess. 2006 Jan;10(1):iii-iv, ix-xi, 1-160. doi: 10.3310/hta10010. Health Technol Assess. 2006. PMID: 16409879
References
-
- Scheff SW, Price DA, Schmitt FA, et al. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007. May 1; 68: 1505–1508. DOI: 10.1212/01.wnl.0000260698.46517 - PubMed
-
- Ross CA and Poirier MA.. Protein aggregation and neurodegenerative disease. Nat Med 2004; 10(Suppl): S10–S17. - PubMed
-
- Emerit J, Edeas M and Bricaire F.. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother 2004; 58(1): 39–46. DOI: 10.1016/j.biopha.2003.11.004 - PubMed
-
- Streit WJ. Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Bull 2005; 48(2): 234–239. DOI: 10.1016/j.brainresrev.2004.12.013 - PubMed
LinkOut - more resources
Full Text Sources

