G Protein-Coupled Receptor 30 Attenuates Neuronal Pyroptosis Induced by Subarachnoid Hemorrhage
- PMID: 40726812
- PMCID: PMC12303633
- DOI: 10.1155/mi/3585885
G Protein-Coupled Receptor 30 Attenuates Neuronal Pyroptosis Induced by Subarachnoid Hemorrhage
Abstract
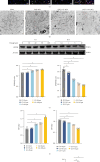
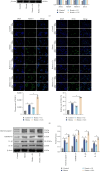
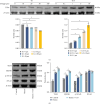
Background: Pyroptosis is implicated as a pathogenic mechanism in early brain injury (EBI) after subarachnoid hemorrhage (SAH). This study aimed to investigate the regulatory role of G protein-coupled receptor 30 (GPR30) in neuronal pyroptosis during SAH. Methods: SAH was induced in rats via intravascular perforation and hemin-treated neurons modeled SAH in vitro. GPR30 agonist G1 and antagonist G15 were administered to assess functional impacts. Neurological deficits (Garcia score), SAH severity, and cerebral edema (brain water content) were evaluated. Pyroptotic markers (cleaved caspase-1, gasdermin D (GSDMD)-N, interleukin (IL)-1β, and IL-18) were quantified. Inflammasome activation (NLRC4 and IFI16) and Toll-like receptor 4/nuclear factor kappa-B (TLR4/NF-κB) signaling were analyzed via immunofluorescence (IF) and immunoblotting. The TLR4 antagonist LPS-RS (lipopolysaccharide from Rhodobacter sphaeroides) was applied to validate pathway involvement. Results: GPR30 expression increased post-SAH. G15 exacerbated hemorrhage severity, neurological deficits, and cerebral edema, whereas G1 modestly attenuated SAH. G15 upregulated pyroptotic markers, enhanced neuronal pyroptosis, and activated NLRC4/IFI16 inflammasomes. Concurrently, G15 stimulated TLR4/MyD88 expression and NF-κB phosphorylation. Conversely, G1 suppressed pyroptosis, inactivated inflammasomes, and inhibited TLR4/NF-κB signaling. LPS-RS cotreatment reversed G15-induced pyroptotic and inflammatory cascades. Conclusion: GPR30 mitigates NLRC4- and IFI16-driven neuronal pyroptosis in SAH by modulating TLR4/NF-κB signaling. Pharmacological targeting of GPR30 represents a promising therapeutic strategy to ameliorate SAH-associated brain injury.
Keywords: IFI16; NLRC4; TLR4/NF-κB; inflammasome; pyroptosis; subarachnoid hemorrhage.
Copyright © 2025 Jun Peng et al. Mediators of Inflammation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
GPR30 Inhibits Neuronal Apoptosis After Subarachnoid Hemorrhage by Activating the Wnt/β-Catenin Pathway in a m6A-dependent Manner.Mol Neurobiol. 2025 Aug;62(8):9638-9650. doi: 10.1007/s12035-025-04867-9. Epub 2025 Mar 25. Mol Neurobiol. 2025. PMID: 40131697
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Complement Molecule C3a Exacerbates Early Brain Injury After Subarachnoid Hemorrhage by Inducing Neuroinflammation Through the C3aR-ERK-P2X7-NLRP3 Inflammasome Signaling Axis.Inflammation. 2025 Aug;48(4):1791-1810. doi: 10.1007/s10753-024-02155-7. Epub 2024 Nov 11. Inflammation. 2025. PMID: 39528767
-
Activation of GPR30 with G1 attenuates neuronal apoptosis via src/EGFR/stat3 signaling pathway after subarachnoid hemorrhage in male rats.Exp Neurol. 2019 Oct;320:113008. doi: 10.1016/j.expneurol.2019.113008. Epub 2019 Jul 8. Exp Neurol. 2019. PMID: 31295444
-
Inhibition of acid-sensing receptor GPR4 attenuates neuronal ferroptosis via RhoA/YAP signaling in a rat model of subarachnoid hemorrhage.Free Radic Biol Med. 2024 Nov 20;225:333-345. doi: 10.1016/j.freeradbiomed.2024.10.273. Epub 2024 Oct 10. Free Radic Biol Med. 2024. PMID: 39393553
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

