3D in vitro modeling of neural microenvironment through a multi-scaffold assembly approach
- PMID: 40727076
- PMCID: PMC12303067
- DOI: 10.1016/j.mtbio.2025.102086
3D in vitro modeling of neural microenvironment through a multi-scaffold assembly approach
Abstract
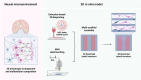
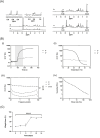
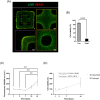
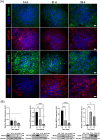
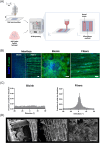
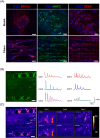
The engineering of in vitro 3D cell culture systems has emerged as promising approach to model central nervous system (CNS) intricacy with increasing physiological relevance. The fabrication of artificial microenvironments that closely resemble nervous tissue composition and architecture has provided useful substrates to promote neural cell growth and maturation under in vivo-like conditions; however, despite significant progress has been made in tissue mimicry, directing neural cell arrangement and connectivity in a controlled 3D environment remains extremely challenging. Here, we propose a novel approach that combines different biomaterials and biofabrication techniques to develop a multi-scaffold system mimicking distinctive features of the nervous tissue. Extrusion-based 3D bioprinting is employed to accurately position neural stem cells (NSCs) embedded in a gelatin methacryloyl hydrogel onto an aligned microfibrous polycaprolactone structure obtained by melt electrowriting. The hydrogel matrix successfully supports NSC growth within 3D bioprinted constructs, ensuring high cell viability and in situ NSC differentiation into neuronal and glial phenotypes. Additionally, melt electrowriting technology allows the design of a microfibrous scaffold having well-defined geometry and aligned microporosity to replicate the anisotropic characteristics of nervous tissue. The inclusion of such scaffold in the 3D bioprinted system effectively steers neural cell organization in a 3D setting, guiding neural cell elongation in a preferred direction and promoting the establishment of a functional neural network. Our approach can be used to develop more sophisticated multicellular systems, possibly reassembling specific CNS circuits within a biomimetic microarchitecture, thus offering a versatile platform for the investigation of CNS functioning and pathology.
Keywords: 3D bioprinting; Aligned topography; Melt electrowriting; Neural stem cells.
© 2025 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Chiara Tonda-Turo reports financial support was provided by 10.13039/501100024370Ministry of Education and Merit. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Effects of amyloid-β-mimicking peptide hydrogel matrix on neuronal progenitor cell phenotype.Acta Biomater. 2024 Jul 15;183:89-100. doi: 10.1016/j.actbio.2024.05.020. Epub 2024 May 25. Acta Biomater. 2024. PMID: 38801867 Free PMC article.
-
Sheet-based extrusion bioprinting: a new multi-material paradigm providing mid-extrusion micropatterning control for microvascular applications.Biofabrication. 2024 Mar 14;16(2):025032. doi: 10.1088/1758-5090/ad30c8. Biofabrication. 2024. PMID: 38447217 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
References
LinkOut - more resources
Full Text Sources

