Exploring the therapeutic mechanism of itraconazole combined with ritonavir on Candida albicans infection through network pharmacology and molecular docking
- PMID: 40727105
- PMCID: PMC12301349
- DOI: 10.3389/fphar.2025.1578749
Exploring the therapeutic mechanism of itraconazole combined with ritonavir on Candida albicans infection through network pharmacology and molecular docking
Abstract
Background: Autophagy induced by itraconazole and ritonavir was found involved in the pathogenesis of C. albicans. This study was designed to explore the possible molecular mechanism of itraconazole and ritonavir in the treatment of Candida albicans infection through autophagy pathway.
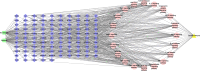
Methods: The overlapping targets of itraconazole and ritonavir, and those-related to C. albicans and autophagy were screened. Then the core targets were identified by protein-protein interaction (PPI) network analysis. Gene enrichment analysis of targets and the drug-target-pathway-disease network was constructed. The interactions between itraconazole, ritonavir and core targets were analyzed by molecular docking and molecular dynamics simulation. Finally, the core target-miRNA interaction network was constructed to predict candidate miRNAs.
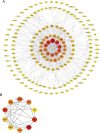
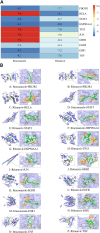
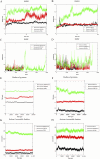
Results: PPI network showed that PIK3R1, RELA, STAT3, HSP90AA1, TP53, JUN, GRB2, EGFR, ESR1 and TNF were potential core targets of autophagy therapy for C. albicans infection with itraconazole and ritonavir. Enrichment analysis showed that the two drugs may regulate the autophagy process through pathways including PI3K-AKT, IL-17, MAPK, Toll-like receptor, JAK-STAT and NF-κB. Molecular docking analysis indicated that itraconazole and ritonavir possess strong binding affinities with the cote target proteins, with binding free energies ranging from -5.6 to -9.5 kcal/mol. Key interactions were identified at the active sites of the targets, suggesting stable ligand-receptor complex formation. Itraconazole docked to PIK3R1 through SER-78 and GLU-82 (-9.3 kcal/mol), and ritonavir docked to PIK3R1 through ASN-85, GLU-1011 and arginine (ARG)-1088 (-7.7 kcal/mol). Molecular dynamics simulation of itraconazole and ritonavir with representative target genes lasted for 100 ns showed the structures of the formed complexes remained stable throughout. Finally, the candidate miRNAs including miR-486-5p, miR-411-5p.1 and miR-296-5p were identified.
Conclusion: Network pharmacological analysis showed a multi-target and multi-pathway molecular mechanism of itraconazole and ritonavir in the treatment of C. albicans infection, and provided a theoretical basis for subsequent studies.
Keywords: Candida albicans; autophagy; itraconazole; network pharmacology; ritonavir.
Copyright © 2025 Feng, Feng, Yang and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Elucidating the Mechanism of Xiaoqinglong Decoction in Chronic Urticaria Treatment: An Integrated Approach of Network Pharmacology, Bioinformatics Analysis, Molecular Docking, and Molecular Dynamics Simulations.Curr Comput Aided Drug Des. 2025 Jul 16. doi: 10.2174/0115734099391401250701045509. Online ahead of print. Curr Comput Aided Drug Des. 2025. PMID: 40676786
-
Understanding mechanisms of Polygonatum sibiricum-derived exosome-like nanoparticles against breast cancer through an integrated metabolomics and network pharmacology analysis.Front Chem. 2025 Jun 6;13:1559758. doi: 10.3389/fchem.2025.1559758. eCollection 2025. Front Chem. 2025. PMID: 40547857 Free PMC article.
-
Study on the mechanism of Shujin Tongluo granules in treating cervical spondylosis based on network pharmacology and molecular docking.Medicine (Baltimore). 2023 Jul 21;102(29):e34030. doi: 10.1097/MD.0000000000034030. Medicine (Baltimore). 2023. PMID: 37478234 Free PMC article.
-
Multi-target Mechanisms of Si-Ni-San on Anxious Insomnia: An Example of Network-pharmacology and Molecular Docking Analysis.Curr Med Chem. 2025;32(13):2640-2663. doi: 10.2174/0109298673299665240924090617. Curr Med Chem. 2025. PMID: 39410900 Free PMC article.
-
Medicine for chronic atrophic gastritis: a systematic review, meta- and network pharmacology analysis.Ann Med. 2023;55(2):2299352. doi: 10.1080/07853890.2023.2299352. Epub 2024 Jan 3. Ann Med. 2023. PMID: 38170849 Free PMC article.
References
-
- Abraham M. J., Murtola T., Schulz R., Páll S., Smith J. C., Hess B., et al. (2015). GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25. 10.1016/j.softx.2015.06.001 - DOI
-
- Alyahya E. M., Alwabsi K., Aljohani A. E., Albalawi R., El-Sherbiny M., Ahmed R., et al. (2023). Preparation and optimization of itraconazole transferosomes-loaded HPMC hydrogel for enhancing its antifungal activity: 2^3 full factorial design. Polym. (Basel) 15 (4), 995. 10.3390/polym15040995 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

