Translational reprogramming under heat stress: a plant's perspective
- PMID: 40727411
- PMCID: PMC12303099
- DOI: 10.1098/rsos.250132
Translational reprogramming under heat stress: a plant's perspective
Abstract
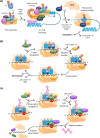
Plants experience dynamic and sometimes extreme fluctuations in temperature on hourly, daily and seasonal scales, which are becoming increasingly challenging as climate change progresses. To maximize fitness and chances of survival, plants continuously adjust their growth, development and physiology to their temperature environment. Changes in protein synthesis are central to these acclimatization processes, enabling rapid and precise modulation of cellular functions. In this review, we discuss the molecular mechanisms driving heat-induced translational reprogramming, integrating insights from animal and yeast systems with current knowledge and emerging hypotheses in plants. We revisit the core stages of translation-initiation, elongation and termination-and the roles of associated translation factors while also exploring emerging areas of interest, including biomolecular condensates, RNA modifications and cis-regulatory elements. Finally, we consider how a deeper understanding of translational control could be harnessed to enhance crop resilience in the face of climate change.
Keywords: heat stress; protein synthesis; temperature sensing; translation; translation factors.
© 2025 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures

Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
Factors that impact on the use of mechanical ventilation weaning protocols in critically ill adults and children: a qualitative evidence-synthesis.Cochrane Database Syst Rev. 2016 Oct 4;10(10):CD011812. doi: 10.1002/14651858.CD011812.pub2. Cochrane Database Syst Rev. 2016. PMID: 27699783 Free PMC article.
-
Fabricating mice and dementia: opening up relations in multi-species research.In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. PMID: 40690569 Free Books & Documents. Review.
References
-
- Benet M, Miguel A, Carrasco F, Li T, Planells J, Alepuz P, Tordera V, Pérez-Ortín JE. 2017. Modulation of protein synthesis and degradation maintains proteostasis during yeast growth at different temperatures. Biochim. Et Biophys. Acta Gene Regul. Mech. 1860, 794–802. ( 10.1016/j.bbagrm.2017.04.003) - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

