Role of the TLR signaling pathway in the pathogenesis of glioblastoma multiforme with an emphasis on immunotherapy
- PMID: 40727443
- PMCID: PMC12302769
- DOI: 10.1016/j.bbrep.2025.102149
Role of the TLR signaling pathway in the pathogenesis of glioblastoma multiforme with an emphasis on immunotherapy
Abstract
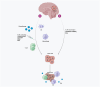
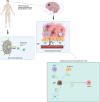
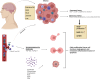
The most malignant brain tumor, glioblastoma multiforme (GBM), has a high mortality rate. Recently, translational elements in GBM therapy have emerged as novel therapeutic strategies in addition to conventional treatment methods. In this way, Toll-like receptor (TLR), PI3K/Akt/mTOR, MAPK/ERK, NOTCH, and other signaling pathways have recently become some of the main signaling pathways in brain tumors. The immunological reactions to brain tumors are mediated by these mechanisms. A family of proteins known as TLRs is essential to the natural defense mechanism because it can identify and react to infections and other danger signals. TLRs have dual functions in the glioma microenvironment including that they can initially activate the innate and adaptive immune responses that support antitumor activity and secondly, their activation can also contribute to tumor progression by promoting inflammation and immune evasion, as they are expressed on both immune cells and tumor cells. TLR agonists are receiving more attention in the treatment of glioma because some of them have demonstrated survival benefits in clinical studies when used in conjunction with immunotherapy, chemotherapy, radiation therapy, and immune checkpoint inhibitors. The most exciting use of TLR agonists is that they can be used as immunomodulators to avoid dose accumulation, boost the efficiency of other therapies, and, by upregulating PD-1, reinforce delayed immune checkpoint resistance against PD-1/PD-L1 inhibition. Therefore, the use of TLR agonists can lead to PD-L1 overexpression, which in turn enhances the efficacy of checkpoint inhibitors and triggers potent anticancer immune responses. In this article, we describe the function of the TLR signaling system, the cellular and molecular elements contributing to the etiology of glioblastoma multiforme, the connection between TLRs and glioma, and their significance for immunotherapy.
Keywords: Immunotherapy; PD-1/PD-L1; TLR signaling pathway; The pathogenesis of glioblastoma.
© 2025 The Authors.
Conflict of interest statement
Hope you are doing well. According to the submission of our manuscript which is entitled: “Role of TLR signaling pathway in the pathogenesis of glioblastoma multiforme with emphasis on immunotherapy” In this journal, all authors declare that there is no conflict of interest and also all the ethical standards considered carefully. Remarkably, all the authors studied and confirmed the final edited version of this manuscript. We hope that the manuscript will receive a fair review and will hear from you positively soon.
Figures



Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
References
-
- Chen X., Zhang Y., Fu Y. The critical role of toll-like receptor-mediated signaling in cancer immunotherapy. Medicine in Drug Discovery. 2022;14
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

