Integration of co-culture conditions and 3D gelatin methacryloyl hydrogels to improve human-induced pluripotent stem cells-derived cardiomyocytes maturation
- PMID: 40727646
- PMCID: PMC12301886
- DOI: 10.3389/fbioe.2025.1576824
Integration of co-culture conditions and 3D gelatin methacryloyl hydrogels to improve human-induced pluripotent stem cells-derived cardiomyocytes maturation
Abstract
Introduction: Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) represent an excellent alternative to animals for in vitro cardiac studies. However, their immature fetal phenotype represents an important limit to consider. Approaches proposed to overcome this issue are based on better reproducing the in vivo native CMs microenvironment. In the present work, a biomimetic environment to enhance hiPSC-CMs maturation was developed by combining a 14-day co-culture of hiPSC-CMs and Human Coronary Artery Endothelial cells (HCAECs) in a 3D Gelatin Methacryloyl (GelMA) hydrogel system.
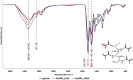
Methods: Chemical characterization of custom-synthesized GelMA was performed through Attenuated Total Reflectance Fourier Transformed Infrared (ATR-FTIR) and proton Nuclear Magnetic Resonance (1H NMR) spectroscopies. GelMA degree of methacryloylation (DoM) was estimated through the ninhydrin colorimetric assay. Then, hydrogels were prepared by solubilizing GelMA in presence of phenyl-2,4,6-trimethyl-benzoyl phosphinate (LAP) as photoinitiator (0.05% w/v) and photo-rheological tests were carried out to investigate the photo-polymerization process (at 365 nm, 10 mW/cm2) and the mechanical properties of the resulting gels. Hydrogel swelling ratio was also monitored up to 5 days of incubation in aqueous medium at 37°C. The maturation phenotype was achieved by co-culturing hiPSC-CMs with HCAECs in the 3D model composed of GelMA with around 96% DoM, solubilized at 5% w/v concentration in cell culture medium, added with LAP and crosslinked by UV light (40 s). The expression of specific cardiac maturation markers was investigated through Real-Time PCR (RT-PCR). Omics analyses were carried out to compare terms of biological processes, cellular components, and molecular functions between the 3D model here presented and a classical 2D monoculture of hiPSC-CMs.
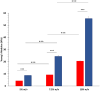
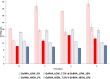
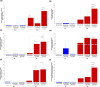
Results: GelMA was successfully synthesized with two different DoMs (i.e., 30%-40% and 96%-97%) and used to prepare hydrogels at 5%, 7.5% and 10% w/v concentrations. Both GelMA DoM and hydrogel concentration appeared as tuning parameters of gel behavior in aqueous environment at 37°C and mechanical properties, with Young's Modulus of photo-cured gels ranging between ca. 4 and 55 kPa. Within this plethora, photo-cured gels prepared from GelMA with ca. 96% DoM solubilized at 5% w/v concentration showed prolonged stability over time and E value (8.70 ± 0.12 kPa) similar to the native cardiac tissue and were thus selected to design bioengineered cardiac tissue models upon hiPSC-CMs and HCAECs loading. A direct comparison with the classical 2D monoculture of hiPSC-CMs highlighted the improved maturation profile achieved by hiPSC-CMs in the 3D GelMA system, as demonstrated by the higher expression of cardiac maturation markers (TNNT2, ACTN2, Myl2, MYH 7, CX43 and PPAR-α), in association with proteomics and transcriptomics data, that showed the modulation of specific biological pathways related to cardiac differentiation and contraction processes in the 3D system. A more in-depth investigation of cell health and function also suggested a higher viability and less suffering condition for cells co-cultured in the 3D hydrogel.
Conclusion: Our results demonstrated that the 3D bioengineered model proposed here represents a good replica of the native cardiac tissue environment, improving the hiPSC-CMs maturation profile, thus opening the opportunity for its application in disease modeling and toxicological screening studies.
Keywords: 3D model; gelatin methacryloyl; hiPSC-CMs culture; hiPSCs differentiation; hydrogels.
Copyright © 2025 Gisone, Boffito, Persiani, Pappalardo, Ceccherini, Alliaud, Cabiati, Laurano, Guiducci, Caselli, Ragusa, Cassino, Ippolito, Del Ry, Sartori, Cecchettini, Fernández-Arroyo, Ciardelli and Vozzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

