Immune Development in Early Life (IDEaL) longitudinal cohort study protocol: Identifying biomarkers of vaccine responsiveness, respiratory infection, and asthma
- PMID: 40727648
- PMCID: PMC12302638
- DOI: 10.1016/j.jacig.2025.100517
Immune Development in Early Life (IDEaL) longitudinal cohort study protocol: Identifying biomarkers of vaccine responsiveness, respiratory infection, and asthma
Abstract
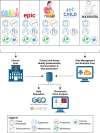
Background: Early-life immune development is a critical factor in predicting the risk of childhood respiratory infections, asthma, and poor vaccine responses. Identifying immune endotypes that predispose children to these conditions could lead to the development of predictive biomarkers and early interventions, potentially improving long-term health outcomes. The IDEaL (Immune Development in Early Life)-Rome prospective pediatric cohort, based at Children's Hospital Bambino Gesù (Rome, Italy), is part of a National Institutes of Health/National Institute of Allergy and Infectious Diseases-supported longitudinal observational study.
Objectives: To identify molecular biomarkers associated with increased susceptibility to respiratory infections, asthma, and poor vaccine responsiveness in early childhood. The study aims to establish predictive immune profiles that could guide interventions to redirect harmful immune trajectories.
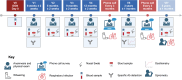
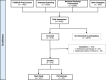
Methods: Mothers are approached during pregnancy prospectively and eligible infants are enrolled at delivery. The study includes 6 planned visits up to 5 years of age. Biosamples (blood, stool, nasal swabs, and cord blood for a subset) were collected at each visit for multiomic data (cytokines, proteomics, microbiome), alongside clinical data on vaccination, infections, and wheezing.
Results: The study included 273 participants (100% enrollment completion rate). Over 2 years, clinical and multiomic data were integrated to investigate immune trajectories related to clinical outcomes. Specific data on the outcomes will be provided in future reports as longitudinal analysis continues.
Conclusions: The IDEaL-Rome cohort study seeks to identify biomarkers predicting immune development trajectories. These findings could enable early interventions to redirect harmful immune trajectories in infancy and improve health outcomes, though further studies are required to validate biomarkers and refine predictive models for clinical application.
Keywords: Vaccine responsiveness; asthma; endotypes; immune development; multiomic profiling; respiratory infection.
© 2025 The Author(s).
Conflict of interest statement
This study was supported by the 10.13039/100000002US National Institutes of Health 10.13039/100000060National Institute of Allergy and Infectious Diseases Immune Development in Early Life (IDEaL) award U19AI168643. This work was also partially supported by the Italian 10.13039/100009647Ministry of Health and 10.13039/100021016Istituto di Ricovero e Cura a Carattere Scientifico “Bambino Gesù” through a 5 × 1000 grant to DA and PP. Disclosure of potential conflict of interest: O. Levy is a named inventor on patents held by Boston Children’s Hospital relating to vaccine adjuvants and in vitro systems that model vaccine action, a cofounder of ARMR Sciences (formerly Ovax, Inc), and a consultant to ARMR Sciences and GlaxoSmithKline (GSK). His laboratory receives sponsored research support from GSK. J.L. Su is a named inventor on patents held by Brigham and Women’s Hospital related to Aging Biomarkers. The rest of the authors declare that they have no relevant conflicts of interest.
Figures

References
-
- Toivonen L., Karppinen S., Schuez-Havupalo L., Teros-Jaakkola T., Vuononvirta J., Mertsola J., et al. Burden of recurrent respiratory tract infections in children: a prospective cohort study. Pediatr Infect Dis J. 2016;35:e362–e369. - PubMed
LinkOut - more resources
Full Text Sources

