Differential Modulation of Copper(II) Interactions with the 18-22 Coordinating Amylin Fragment by the Geometric Isomers of a New Nicotinoyl Hydrazone: A First Study
- PMID: 40727756
- PMCID: PMC12290932
- DOI: 10.1021/acsomega.5c04850
Differential Modulation of Copper(II) Interactions with the 18-22 Coordinating Amylin Fragment by the Geometric Isomers of a New Nicotinoyl Hydrazone: A First Study
Abstract
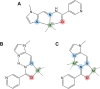
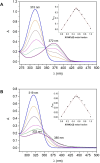
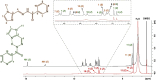
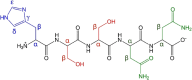
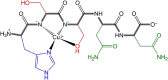
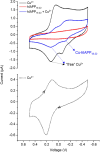
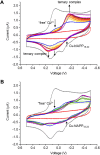
Type 2 diabetes mellitus is a multifactorial disease associated with insulin resistance and pancreatic β-cell dysfunction. Interestingly, this disease has also been associated with the aggregation of islet amyloid polypeptide (IAPP or amylin). This peptide can bind to physiological metal ions such as copper-(II), which enhances its aggregation and induces oxidative stress. For this reason, the use of moderate chelators constitutes a compelling potential therapeutic strategy. In this work, we synthesized and characterized the geometric isomers of a new compound, 1-methylimidazole-2-carboxaldehyde nicotinoyl hydrazone (X1NIC), aiming to evaluate their differential interactions with copper-(II) and the hIAPP18-22 peptide fragment. Both compounds demonstrated high aqueous stability and adequate lipophilicity for biological membrane crossing. Coordination studies revealed that both ML and ML2 complexes can be obtained in solution for the tridentate ligand X1NIC-( E ), with the former being more stable. On the other hand, the bidentate ligand X1NIC-( Z ) only forms ML species. Both isomers effectively set up ternary complexes with Cu2+ and hIAPP18-22, altering the redox behavior of the copper-peptide system. These results, obtained through cyclic voltammetry experiments, suggest a protective effect of the ligands with respect to metal-induced oxidative stress. This study constitutes the first comparative report on the coordination and reactivity of geometric isomers of a bioactive N-acylhydrazone, and the findings described herein highlight this class of compounds as promising chemical tools for the modulation of abnormal metal-peptide interactions implicated in type 2 diabetes pathogenesis.
© 2025 The Authors. Published by American Chemical Society.
Figures


References
-
- Enoki S., Mitsukawa T., Takemura J., Nakazato M., Aburaya J., Toshimori H., Matsukara S.. Plasma islet amyloid polypeptide levels in obesity, impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabetes Res. Clin Pract. 1992;15(1):97–102. doi: 10.1016/0168-8227(92)90074-2. - DOI - PubMed
- Kautzky-Willer A., Thomaseth K., Ludvik B., Nowotny P., Rabensteiner D., Waldhäusl W., Pacini G., Prager R.. Elevated islet amyloid pancreatic polypeptide and proinsulin in lean gestational diabetes. Diabetes. 1997;46(4):607–614. doi: 10.2337/diab.46.4.607. - DOI - PubMed
LinkOut - more resources
Full Text Sources
