Analysis of the Photocatalytic Activity and Adsorption of CuS Nanoparticles Synthesized by Chemical Route for the Degradation of Organic Contaminants
- PMID: 40727768
- PMCID: PMC12290959
- DOI: 10.1021/acsomega.5c02366
Analysis of the Photocatalytic Activity and Adsorption of CuS Nanoparticles Synthesized by Chemical Route for the Degradation of Organic Contaminants
Abstract
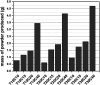
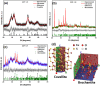
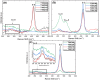
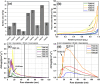
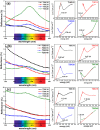
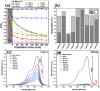
The treatment of industrial and domestic wastewater is an urgent environmental need. In this context, the photocatalytic activity of semiconductors offers a promising route for degrading organic contaminants. CuS nanoparticles were chemically synthesized using thiourea and copper sulfate in varying concentrations to investigate how precursor ratios affect the chemical composition, structural and morphological features, and optical-electronic properties. The photocatalytic degradation of methylene blue under low-power visible light (10 W), without H2O2 and using a low catalyst dose, showed promising results. Samples with lower sulfate content reached ∼78% degradation, while those with 0.20 M thiourea and 0.15-0.20 M sulfate achieved up to 99%. Mesoporous and macroporous structures (3.85-50 nm) promoted adsorption without hindering photocatalytic efficiency, indicating that, in certain samples, the combined morphological and electronic features enhanced dye removal.
© 2025 The Authors. Published by American Chemical Society.
Figures










References
-
- Iqbal T., Jameel M. A., Farooq M., Mansha M. S., Afsheen S., Al-Zaqri N., El-marghany A., Warad I., Khan H.. Facile hydrothermal synthesis of Cu-doped MoS2 nanomaterial: a potential photocatalyst for degradation of MB dye. Opt. Quantum Electron. 2024;56:156. doi: 10.1007/s11082-023-05759-9. - DOI
-
- Qin, N. ; Wei, W. ; Huang, C. ; Mi, L. , An Efficient Strategy for the Fabrication of CuS as a Highly Excellent and Recyclable Photocatalyst for the Degradation of Organic Dyes, Catalysts 2020. 10 (2020) 40. 10.3390/catal10010040. - DOI
-
- Sarker B., Keya K. N., Mahir F. I., Nahiun K. M., Shahida S., Khan R. A.. Surface and Ground Water Pollution: Causes and Effects of Urbanization and Industrialization in South Asia. Sci. Rev. 2021;7:32–41. doi: 10.32861/sr.73.32.41. - DOI
-
- Isac L., Cazan C., Andronic L., Enesca A.. CuS-Based Nanostructures as Catalysts for Organic Pollutants Photodegradation. Catalysts. 2022;12:1135. doi: 10.3390/catal12101135. - DOI
-
- Mousavi-Kamazani M., Zarghami Z., Salavati-Niasari M.. Facile and Novel Chemical Synthesis, Characterization, and Formation Mechanism of Copper Sulfide (Cu2S, Cu2S/CuS, CuS) Nanostructures for Increasing the Efficiency of Solar Cells. J. Phys. Chem. C. 2016;120:2096–2108. doi: 10.1021/acs.jpcc.5b11566. - DOI
LinkOut - more resources
Full Text Sources
