Orexin/Hypocretin Modulates Neuroinflammatory Response to LPS in a Sex and Brain-Region Specific Manner in Young Rats
- PMID: 40728035
- PMCID: PMC12305754
- DOI: 10.1111/jnc.70175
Orexin/Hypocretin Modulates Neuroinflammatory Response to LPS in a Sex and Brain-Region Specific Manner in Young Rats
Abstract
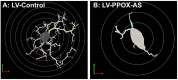
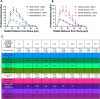
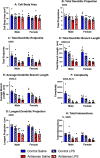
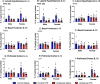
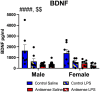
Neuroinflammation has emerged as a contributing mechanism in age-related cognitive decline (ARCD), Parkinson's disease (PD), obesity, sleep disorders, and autoimmune disorders. Orexin/hypocretin, a neuropeptide expressed in the lateral hypothalamus (LH), has well-established roles in homeostatic processes, such as energy metabolism, food intake, sleep, and wakefulness. Our laboratory and others have shown that orexin expression decreases with age, and this age-related orexin decline is exacerbated in disease states. Additionally, it has recently been shown that orexin possesses anti-inflammatory and neuroprotective properties. Based on these observations, we hypothesize that orexin is modulating neuroinflammation in brain regions that are critical in the development of ARCD. To test this hypothesis, we used lentiviral gene transfer to downregulate orexin expression in male and female young rats to mimic age-related orexin deficiency and examined neuroinflammatory responses to peripheral administration of lipopolysaccharide (LPS). We found a significant reduction of basal forebrain (BF) microglial complexity and plasma BDNF in both males and females following orexin downregulation. Notably, orexin downregulation blocked the capacity of the neuroinflammatory system to respond to LPS. These results demonstrate that neuroinflammatory responses are dependent on orexin signaling, and this system becomes dysfunctional in aging in a sex-dependent manner.
Keywords: aging; brain‐derived neurotrophic factor; cytokines; microglia; orexin/hypocretin.
© 2025 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ashdown, H. , Poole S., Boksa P., and Luheshi G. N.. 2007. “Interleukin‐1 Receptor Antagonist as a Modulator of Gender Differences in the Febrile Response to Lipopolysaccharide in Rats.” American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 292, no. 4: R1667–R1674. 10.1152/ajpregu.00274.2006. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

