Respiratory and Metabolic Effects of Active Expiration in Freely Behaving Rats
- PMID: 40728246
- PMCID: PMC12306407
- DOI: 10.1111/apha.70084
Respiratory and Metabolic Effects of Active Expiration in Freely Behaving Rats
Abstract
Aim: Exposure to low oxygen (hypoxia) or high carbon dioxide levels (hypercapnia) leads to a compensatory increase in pulmonary ventilation. Among the motor changes supporting the respiratory responses is the recruitment of abdominal expiratory muscles (ABD), which can enhance expiratory airflow or alter the duration of the expiratory phase. In this study, we assessed the impact of ABD recruitment on metabolic, motor, and ventilatory parameters in unanesthetized, freely behaving animals.
Methods: Sprague-Dawley Holtzman male adult rats (n = 7) were instrumented to perform simultaneous recordings of pulmonary ventilation, body temperature, diaphragmatic and ABD activities, and O2 consumption during exposure (20-30 min) to various levels of hypoxia (12%-8% O2) and hypercapnia (3%-7% CO2).
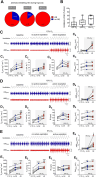
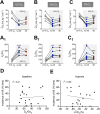
Results: Hypoxia or hypercapnia exposure evoked active expiration (AE); however, ABD recruitment did not occur during the entire exposure period, displaying an intermittent profile. The occurrence of AE during hypoxia and hypercapnia conditions was linked to additional increases in tidal volume when compared to periods without ABD activity (p < 0.05) and showed no associations with changes in diaphragmatic burst amplitude. Analyses of flow-like patterns suggested that AE during hypoxia recruited expiratory reserve volume during late expiration, while under hypercapnia, it accelerated lung emptying and increased the expiratory flow peak during post-inspiration. AE was also associated with increased oxygen consumption and did not improve air convection requirement.
Conclusion: AE enhances pulmonary ventilation during hypoxia and hypercapnia primarily by increasing tidal volume. However, this motor behavior may also affect other mechanical aspects of the respiratory system to improve alveolar ventilation and gas exchange.
Keywords: active expiration; breathing; hypercapnia; hypoxia; pulmonary ventilation.
© 2025 The Author(s). Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Ventilator Management(Archived).2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28846232 Free Books & Documents.
-
Acute intermittent hypercapnic-hypoxia elicits central neural respiratory motor plasticity in humans.J Physiol. 2022 May;600(10):2515-2533. doi: 10.1113/JP282822. Epub 2022 Apr 28. J Physiol. 2022. PMID: 35348218 Free PMC article.
-
Non-invasive ventilation for cystic fibrosis.Cochrane Database Syst Rev. 2017 Feb 20;2(2):CD002769. doi: 10.1002/14651858.CD002769.pub5. Cochrane Database Syst Rev. 2017. PMID: 28218802 Free PMC article.
-
Hypercapnia impacts neural drive and timing of diaphragm neuromotor control.J Neurophysiol. 2024 Dec 1;132(6):1966-1976. doi: 10.1152/jn.00466.2024. Epub 2024 Nov 16. J Neurophysiol. 2024. PMID: 39548981
-
Positioning for acute respiratory distress in hospitalised infants and children.Cochrane Database Syst Rev. 2022 Jun 6;6(6):CD003645. doi: 10.1002/14651858.CD003645.pub4. Cochrane Database Syst Rev. 2022. PMID: 35661343 Free PMC article.
References
-
- Dutschmann M., Jones S. E., Subramanian H. H., Stanic D., and Bautista T. G., “The Physiological Significance of Postinspiration in Respiratory Control,” Progress in Brain Research 212 (2014): 113–130. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

