Candesartan Mitigates Perioperative Neurocognitive Disorders by Modulating Hypertension-Linked Neuroinflammatory Factor
- PMID: 40728591
- PMCID: PMC12307533
- DOI: 10.1007/s11064-025-04499-4
Candesartan Mitigates Perioperative Neurocognitive Disorders by Modulating Hypertension-Linked Neuroinflammatory Factor
Abstract
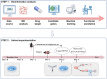
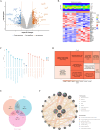
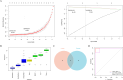
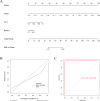
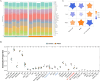
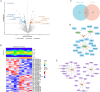
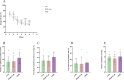
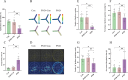
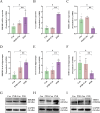
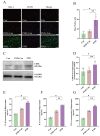
Perioperative neurocognitive disorders (PND) are linked to neuroinflammation, a key factor in hypertension, but their causal relationship is underexplored. This study aims to investigate whether hypertension is a risk factor for PND, identify related neuroinflammatory targets, and determine if the angiotensin receptor blockers (ARBs) candesartan can improve cognitive function in PND mouse models by modulating these targets. This study identified hypertension as a risk factor for cognitive dysfunction (OR = 1.0767, P = 0.0057) through Mendelian randomization (MR) analysis. Subsequently, bioinformatics techniques were employed to identify the neuroinflammatory targets associated with hypertension for ARBs. Differential analysis revealed Bdkrb1, Ccr1, and Thbs1 were PND biomarkers associated with hypertension, confirmed by machine learning and receiver operating characteristic (ROC) analysis (area under the curve (AUC) > 0.9). Immune infiltration showed Thbs1 positively correlated with MoDC cells (r = 0.70), while Bdkrb1 negatively correlated with Plasma cells (r = -0.75). In the PND mouse model, we assessed whether candesartan could inhibit the onset of neuroinflammation by modulating the targets identified through our screening process. Molecular experiments, including RT-qPCR, Western blotting, immunofluorescence, and ELISA, analyzed gene expression and neuroinflammatory changes in the hippocampus. In a PND mouse model, candesartan improved cognitive function, reducing escape latency and increasing spontaneous alternation rates. Molecular analysis demonstrated candesartan downregulated Bdkrb1 and Ccr1 expression while upregulating Thbs1 in the hippocampus. Additionally, candesartan reduced IL-1β, IL-6, TNF-α levels and microglial activation, highlighting its anti-inflammatory and neuroprotective effects in PND. In conclusion, candesartan improved cognitive function in PND mice by modulating Bdkrb1, Ccr1, and Thbs1, reducing neuroinflammation, and targeting hippocampal immune responses, highlighting its therapeutic potential for PND.
Keywords: Candesartan; Hypertension; Inflammation; Mendelian randomization; Perioperative neurocognitive disorders.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: The present study was approved by the Laboratory Animal Welfare and Ethics Committee of the Affiliated Hospital of Zunyi Medical University, with the number: zyfy-an-2024-0570. Competing Interests: The authors declare no competing interests.
Figures

Similar articles
-
Gamma Oscillation Disruption Induced By Microglial Activation Contributes to Perioperative Neurocognitive Disorders in Aged Mice.J Mol Neurosci. 2025 Aug 2;75(3):97. doi: 10.1007/s12031-025-02380-1. J Mol Neurosci. 2025. PMID: 40753364
-
Comparative clinical- and cost-effectiveness of candesartan and losartan in the management of hypertension and heart failure: a systematic review, meta- and cost-utility analysis.Int J Clin Pract. 2011 Mar;65(3):253-63. doi: 10.1111/j.1742-1241.2011.02633.x. Epub 2011 Feb 1. Int J Clin Pract. 2011. PMID: 21284790
-
Inhibiting endoplasmic reticulum stress alleviates perioperative neurocognitive disorders by reducing neuroinflammation mediated by NLRP3 inflammasome activation.CNS Neurosci Ther. 2024 Oct;30(10):e70049. doi: 10.1111/cns.70049. CNS Neurosci Ther. 2024. PMID: 39432407 Free PMC article.
-
Non-HLA angiotensin-type-1 receptor autoantibodies mediate the long-term loss of grafted neurons in Parkinson's disease models.Stem Cell Res Ther. 2024 May 12;15(1):138. doi: 10.1186/s13287-024-03751-y. Stem Cell Res Ther. 2024. PMID: 38735991 Free PMC article.
-
Microglial Modulation as a Therapeutic Avenue for Perioperative Neurocognitive Disorders: Unveiling Pathophysiological Mechanisms and Clinical Implications.CNS Neurosci Ther. 2025 Jul;31(7):e70481. doi: 10.1111/cns.70481. CNS Neurosci Ther. 2025. PMID: 40678838 Free PMC article. Review.
References
-
- Needham MJ, Webb CE, Bryden DC (2017) Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth 119:i115–i125. 10.1093/bja/aex354 - PubMed
MeSH terms
Substances
Grants and funding
- HZ-2021-89/Zunyi Science and Technology Cooperation Program Project
- HZ-2023-242/Zunyi Science and Technology Cooperation Program Project
- HZ-2023-226/Zunyi Science and Technology Cooperation Program Project
- rc220240404/Zunyi Medical University's "12345" Future Talent Cultivation Plan
- qiankehe foundation-zk[2024] general 290/Guizhou Provincial science and technology plan project
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

