Per- and Polyfluoroalkyl Substances (PFAS) Enhance Cholesterol Accumulation and Dysregulate Inflammatory Responses in Macrophages
- PMID: 40728694
- PMCID: PMC12432094
- DOI: 10.1007/s12012-025-10048-w
Per- and Polyfluoroalkyl Substances (PFAS) Enhance Cholesterol Accumulation and Dysregulate Inflammatory Responses in Macrophages
Abstract
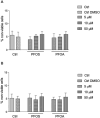
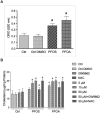
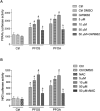
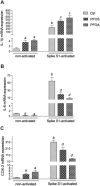
Epidemiological studies and in vivo animal models have shown that exposure to PFAS can lead to cardiovascular toxicity and promote atherosclerosis. In this study, we explored the effects of PFOA and PFOS exposure on lipid accumulation in macrophages and analyzed critical markers of foam cell formation, which are early precursors of atherosclerotic lesions. Our results demonstrate that PFOS and PFOA enhance lipid and cholesterol accumulation in human U937-derived macrophages, which is characteristic of foam cells. PFOS and PFOA induced the activity of the peroxisome proliferator-activated receptor gamma (PPARγ) and treatment with a PPARγ antagonist partly reversed the accumulation of lipids after PFAS exposure. Furthermore, the results show that PFOS and PFOA activate (NF)-erythroid-derived 2 (E2)-related factor 2 (Nrf2) and induce markers of oxidative stress. Gene expression analysis revealed that mRNA levels of interleukin-1β (IL-1β) and plasminogen activator inhibitor-2 (PAI-2) were upregulated in a time- and concentration-dependent manner in PFOS- and PFOA-treated macrophages. The expression of other key atherosclerosis-related enzymes, including cytochrome P450 8B1 (CYP8B1) and lanosterol synthase (LSS), was downregulated, whereas the expression of cyclooxygenase 2 (COX-2) and aldo-keto reductase family 1 member C3 (AKR1C3) was induced by PFOS and PFOA. Additionally, elevated levels of matrix metalloproteinases (MMP)-1 and MMP-12 were found in PFOS- and PFOA-treated cells, which were associated with increased cell migration. Furthermore, PFOS and PFOA enhanced the expression of IL-1β when macrophages were activated; however, elevated levels of IL-6 and COX-2 in activated macrophages were repressed by PFOS and PFOA. Together, the findings indicate that PFAS exposure modifies immune responses and promotes lipid accumulation in macrophages, potentially contributing to foam cell and plaque formation in atherosclerosis.
Keywords: Cardiovascular disease; Cytokines; Foam cells; Lipids; Macrophages; PFAS; PPAR; SARS-CoV-2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: The authors declare no competing interests.
Figures



References
-
- Hu, X. C., Tokranov, A. K., Liddie, J., Zhang, X., Grandjean, P., Hart, J. E., Laden, F., Sun, Q., Yeung, L. W. Y., & Sunderland, E. M. (2019). Tap water contributions to plasma concentrations of poly- and perfluoroalkyl substances (PFAS) in a nationwide prospective cohort of U.S. women. Environmental Health Perspectives,127(6), 67006. 10.1289/EHP4093 - PMC - PubMed
-
- Lin, H., Taniyasu, S., Yamazaki, E., Wei, S., Wang, X., Gai, N., Kim, J. H., Eun, H., Lam, P. K. S., & Yamashita, N. (2020). Per- and polyfluoroalkyl substances in the air particles of Asia: Levels, seasonality, and size-dependent distribution. Environmental Science & Technology,54(22), 14182–14191. 10.1021/acs.est.0c03387 - PubMed
-
- Zhou, J., Baumann, K., Mead, R. N., Skrabal, S. A., Kieber, R. J., Avery, G. B., Shimizu, M., DeWitt, J. C., Sun, M., Vance, S. A., Bodnar, W., Zhang, Z., Collins, L. B., Surratt, J. D., & Turpin, B. J. (2021). PFOS dominates PFAS composition in ambient fine particulate matter (PM2.5) collected across North Carolina nearly 20 years after the end of its US production. Environmental Science: Processes and Impacts,23(4), 580–587. 10.1039/D0EM00497A - PubMed
-
- Faust, J. A. (2023). PFAS on atmospheric aerosol particles: A review. Environmental Science: Processes and Impacts,25(2), 133–150. 10.1039/d2em00002d - PubMed
-
- Labadie, P., & Chevreuil, M. (2011). Partitioning behaviour of perfluorinated alkyl contaminants between water, sediment and fish in the Orge river (nearby Paris, France). Environmental Pollution,159(2), 391–397. 10.1016/j.envpol.2010.10.039 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

