Altered reactivity to threatening stimuli in Drosophila models of Parkinson's disease, revealed by a trial-based assay
- PMID: 40728873
- PMCID: PMC12306971
- DOI: 10.7554/eLife.90905
Altered reactivity to threatening stimuli in Drosophila models of Parkinson's disease, revealed by a trial-based assay
Abstract
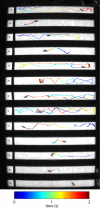
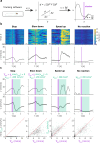
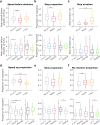
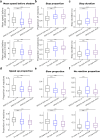
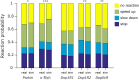
The fruit fly Drosophila melanogaster emerges as an affordable, genetically tractable model of behavior and brain diseases. However, despite the surprising level of evolutionary conservation from flies to humans, significant genetic, circuit-level, and behavioral differences hinder the interpretability of fruit fly models for human disease. Therefore, to allow a more direct fly-versus-human comparison, we surveyed the rarely exploited, rich behavioral repertoire of fruit flies with genetic alterations relevant to Parkinson's disease (PD), including overexpression of human mutant Parkin or α-synuclein proteins and mutations in dopamine receptors. Flies with different genetic backgrounds displayed variable behaviors, including freezing, slowing, and running, in response to predator-mimicking passing shadows used as threatening stimuli in a single-animal trial-based assay. We found that the expression of human mutant Parkin in flies resulted in reduced walking speed and decreased reactivity to passing shadows. Flies with dopamine receptor mutations showed similar alterations, consistent with the motor and cognitive deficits typical in humans with PD. We also found age-dependent trends in behavioral choice during the fly lifespan, while dopamine receptor mutant flies maintained their decreased general reactivity throughout all age groups. Our data demonstrate that single-trial behavioral analysis can reveal subtle behavioral changes in mutant flies that can be used to further our understanding of disease pathomechanisms and help gauge the validity of genetic Drosophila models of neurodegeneration, taking us one step closer to bridging the gap in fly-to-human translation.
Keywords: D. melanogaster; Parkin; Parkinson's disease; dopamine; escape; neuroscience; threat; α-synuclein.
© 2024, Kajtor et al.
Conflict of interest statement
MK, VB, BK, PK, TK, HS, KS, AV, EU, DB, SS, DK, TV, BH No competing interests declared
Figures




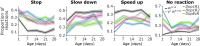

Update of
- doi: 10.1101/2023.08.29.555311
- doi: 10.7554/eLife.90905.1
- doi: 10.7554/eLife.90905.2
References
MeSH terms
Substances
Grants and funding
- LP2024-8/2024/Hungarian Academy of Sciences
- NAP2022-I-1/2022/Hungarian Academy of Sciences
- VEKOP-2.3.2-16-2017-00014/National Research, Development and Innovation Office
- P40 OD018537/OD/NIH HHS/United States
- K147097/National Research, Development and Innovation Office
- NAP-KOLL-2023-1/2023/Hungarian Academy of Sciences
- PD143786/National Research, Development and Innovation Office
- STARTING 150612/National Research, Development and Innovation Office
- 01062/ELKH/MTA-ELTE Genetics Research Group
- K132439/National Research, Development and Innovation Office
- EKA_2022/045-P302-1/Eötvös Loránd University
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

