Living human lung slices for ex vivo modelling of lung cancer
- PMID: 40728884
- PMCID: PMC12487671
- DOI: 10.1172/jci.insight.190703
Living human lung slices for ex vivo modelling of lung cancer
Abstract
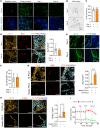
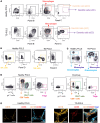
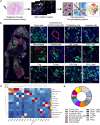
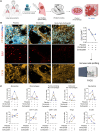
The tumor microenvironment (TME) markedly affects cancer progression, yet traditional animal models do not fully recapitulate the situation in humans. To address this, we developed tumor-derived precision lung slices (TD-PCLS), an ex vivo platform for studying the lung TME and evaluating therapies. TD-PCLS, viable for 8-10 days, preserve the heterogeneity and metabolic activity of primary tumors, as confirmed by seahorse analysis. Using multispectral FACS and phenocycler multiplex imaging, we spatially profiled TME components and cancer cell functionality. Additionally, TD-PCLS revealed patient-specific responses to chemo- and immunotherapies. To complement TD-PCLS, we established tumor-cell-seeded PCLS (TCS-PCLS) by introducing tumor and immune cells into healthy lung slices. This model highlighted macrophage-tumor interactions as critical for tumor cell proliferation, migration, and immune modulation. Together, these platforms provide a robust tool for lung cancer research, enabling precision medicine and advancing therapeutic discovery.
Keywords: Immunology; Immunotherapy; Lung cancer; Oncology.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

