Restoration of Autophagy and Apoptosis in Myelodysplastic Syndromes: The Effect of Azacitidine in Disease Pathogenesis
- PMID: 40728989
- PMCID: PMC12293780
- DOI: 10.3390/cimb47070520
Restoration of Autophagy and Apoptosis in Myelodysplastic Syndromes: The Effect of Azacitidine in Disease Pathogenesis
Abstract
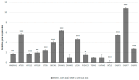
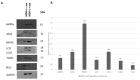
Myelodysplastic syndromes (MDSs) comprise a diverse group of clonal hematopoietic stem cell disorders characterized by ineffective hematopoiesis, cytopenia in the peripheral blood, and an increased risk of transformation into acute myeloid leukemia (AML). Despite extensive research, the mechanisms underlying MDS pathogenesis remain unclear. In the present study, we explored the role of autophagy and apoptosis in the development of MDS and assessed the impact of azacitidine on these processes in vitro. First, we assessed the expression of proteins involved in both autophagic and apoptotic pathways in MDS patients with different prognoses. Furthermore, using the MDS-L cell line as a model, we investigated the in vitro effects of azacitidine treatment on these processes. We report that MDS, irrespective of risk classification, is associated with the dysregulation of autophagy and apoptosis. Notably, azacitidine treatment restored these cellular processes, accompanied by modulation of key signaling phosphoproteins. Overall, these findings provide evidence that impaired autophagy and apoptosis contribute to MDS pathogenesis and that azacitidine helps restore cellular homeostasis by activating both processes. Furthermore, our study highlights the potential therapeutic benefits of targeting these mechanisms and suggests that combining azacitidine with agents that modulate autophagy and apoptosis could enhance the treatment efficacy for MDS patients.
Keywords: apoptosis; autophagy; azacitidine; myelodysplastic syndromes; protein expression profiling.
Conflict of interest statement
Authors Vaia Pliaka and Leonidas Alexopoulos were employed by the company Protavio Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- McDonald L., McCarthy P., Khan M., Hogan P., Kelleher E.C., Murphy P., Quinn J., Desmond R., McHugh J., Strickland M., et al. Should Myelodysplastic Syndromes in Very Old Patients be More Actively Managed? Clinical Characteristics, Management and Outcomes for Patients 85 Years and Older. Blood. 2018;132((Suppl. 1)):5515. doi: 10.1182/blood-2018-99-113290. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

