Dihydroartemisinin Alleviates the Symptoms of a Mouse Model of Systemic Lupus Erythematosus Through Regulating Splenic T/B-Cell Heterogeneity
- PMID: 40728997
- PMCID: PMC12293267
- DOI: 10.3390/cimb47070528
Dihydroartemisinin Alleviates the Symptoms of a Mouse Model of Systemic Lupus Erythematosus Through Regulating Splenic T/B-Cell Heterogeneity
Abstract
Background: Systemic lupus erythematosus (SLE) is a complex autoimmune disease with significant therapeutic challenges. Recent studies suggest that dihydroartemisinin (DHA), a traditional Chinese medicine known for its anti-malarial properties, may be beneficial for SLE treatment, although its precise mechanism remains unclear. This study aimed to investigate the effects of DHA on the cellular composition and molecular events of splenic T cells and B cells in MRL/lpr mice, a widely used SLE model.
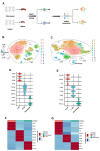
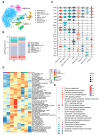
Methods: T cells and B cells isolated from the spleens of three DHA-treated mice and three control mice underwent single-cell RNA sequencing (scRNA-seq) using the 10× Genomics Chromium system. Comprehensive analyses included cell clustering, signaling pathway enrichment, pseudotime trajectory analysis, and cellular communication assessment using unbiased computational methods.
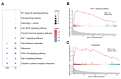
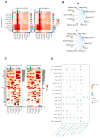
Results: DHA treatment significantly reduced kidney inflammation and altered the proportions of splenic T cells and B cells, particularly decreasing plasma cells. Molecular profiling of effector CD4+ T cells showed a significant reduction in several inflammation-related signaling pathways in DHA-treated mice. Cellular communication analysis indicated altered interactions between effector CD4+ T cells and B cells in MRL/lpr mice after DHA treatment.
Conclusions: Our findings reveal changes in cellular composition and signaling pathways in splenic T cells and B cells of MRL/lpr mice following DHA treatment. DHA may inhibit B-cell differentiation into plasma cells by modulating effector CD4+ T cells, potentially through the regulation of HIF1α and ligand-receptor interactions, enhancing our understanding of DHA's mechanisms in SLE treatment.
Keywords: dihydroartemisinin; lupus; single-cell RNA sequencing; spleen cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Impaired dynamic X-chromosome inactivation maintenance in T cells is a feature of spontaneous murine SLE that is exacerbated in female-biased models.J Autoimmun. 2023 Sep;139:103084. doi: 10.1016/j.jaut.2023.103084. Epub 2023 Jul 1. J Autoimmun. 2023. PMID: 37399593 Free PMC article.
-
Curcumin mitigates systemic lupus erythematosus by suppressing double-negative T cells through cell apoptosis.Cell Immunol. 2025 Aug;414:104996. doi: 10.1016/j.cellimm.2025.104996. Epub 2025 Jun 16. Cell Immunol. 2025. PMID: 40532482
-
Protective Role of Dietary Short-Chain Fatty Acid Propionate against Autoimmune Responses and Pathology of Systemic Lupus Erythematosus in MRL-lpr Mice.J Nutr. 2025 Jul 2:S0022-3166(25)00420-1. doi: 10.1016/j.tjnut.2025.06.031. Online ahead of print. J Nutr. 2025. PMID: 40615087
-
Association between Unsaturated Fatty Acid-Type Diet and Systemic Lupus Erythematosus: A Systematic Review with Meta-Analyses.Nutrients. 2024 Jun 20;16(12):1974. doi: 10.3390/nu16121974. Nutrients. 2024. PMID: 38931327 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

