Colostrum-Derived Exosomal Lactoferrin Promotes Skin Fibroblast Regeneration by Suppressing Inflammatory Responses
- PMID: 40729018
- PMCID: PMC12293444
- DOI: 10.3390/cimb47070549
Colostrum-Derived Exosomal Lactoferrin Promotes Skin Fibroblast Regeneration by Suppressing Inflammatory Responses
Abstract
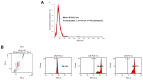
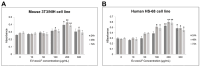
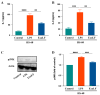
Lactoferrin (LF), a multifunctional glycoprotein found abundantly in bovine colostrum, is known for its regenerative and anti-inflammatory properties. In this study, we investigated the wound healing and immunomodulatory effects of colostrum-derived exosome-encapsulated lactoferrin (EV-exoLF) on dermal fibroblasts. EV-exoLF was isolated and characterized via nanoparticle tracking analysis and flow cytometry. Functional assays demonstrated that EV-exoLF significantly promoted fibroblast viability and migration in both mouse NIH/3T3 and human HS-68 cell lines. Furthermore, EV-exoLF reduced the expression of pro-inflammatory cytokines (IL-1 and IL-6) and phosphorylated JNK in lipopolysaccharide (LPS)-treated fibroblasts. These findings suggest that EV-exoLF not only enhances fibroblast-mediated wound closure but also mitigates inflammation, highlighting its therapeutic potential in skin regeneration. Colostrum-derived exosomal lactoferrin may serve as a promising natural, cell-free strategy for managing inflammatory skin conditions and improving wound healing outcomes.
Keywords: colostrum; cytokines; exosomes; fibroblasts; inflammation; lactoferrin; skin regeneration; wound healing.
Conflict of interest statement
Author Chu-Hsun Cheng, Wen-Chun Kuo was employed by the company Bio-METS Biotech Consulting Co., Ltd., New Taipei City, Taiwan. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Human-induced pluripotent stem cell-derived exosomes promote skin wound healing through activating FGF2-mediated p38 pathway.Mol Cell Biochem. 2025 Jul;480(7):4227-4242. doi: 10.1007/s11010-025-05244-9. Epub 2025 Mar 11. Mol Cell Biochem. 2025. PMID: 40064791
-
Negative pressure wound therapy for surgical wounds healing by primary closure.Cochrane Database Syst Rev. 2022 Apr 26;4(4):CD009261. doi: 10.1002/14651858.CD009261.pub7. Cochrane Database Syst Rev. 2022. PMID: 35471497 Free PMC article.
-
Fibroblast-Derived Extracellular Vesicles Ameliorate the Skin Injury Microenvironment to Promote Wound Healing.Cell Biol Int. 2025 Jul 29. doi: 10.1002/cbin.70063. Online ahead of print. Cell Biol Int. 2025. PMID: 40728022
-
Human umbilical cord mesenchymal stem cell exosomes promote elastin production and acute skin wound healing via TGFβ1-Smad pathway.Mol Cell Biochem. 2025 Jul;480(7):4499-4511. doi: 10.1007/s11010-025-05264-5. Epub 2025 Apr 9. Mol Cell Biochem. 2025. PMID: 40202710 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
Cited by
-
Casein-Based Biomaterials: Fabrication and Wound Healing Applications.Molecules. 2025 Aug 5;30(15):3278. doi: 10.3390/molecules30153278. Molecules. 2025. PMID: 40807458 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

