A Case Report: The Utility of Multimodality Imaging in the Diagnosis of Cardiac Sarcoidosis-Has It Surpassed the Need for a Biopsy?
- PMID: 40729241
- PMCID: PMC12199953
- DOI: 10.3390/reports8010028
A Case Report: The Utility of Multimodality Imaging in the Diagnosis of Cardiac Sarcoidosis-Has It Surpassed the Need for a Biopsy?
Abstract
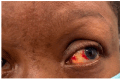
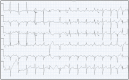
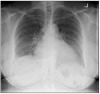
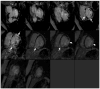
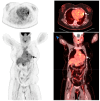
Background and Clinical Significance: Cardiac sarcoidosis (CS) is a rare but life-threatening disorder, occurring in 2-5% of sarcoidosis cases, though post-mortem studies suggest a higher prevalence. It presents diagnostic challenges due to nonspecific symptoms and the low sensitivity of an endomyocardial biopsy. Recent guidelines emphasize multimodal imaging, such as cardiac magnetic resonance imaging (MRI) and positron emission tomography (PET). Given the risk of heart failure (HF) and arrhythmias, early detection is critical. This case highlights the role of non-invasive imaging in diagnosing CS and guiding treatment. Case Presentation: A 54-year-old female with asthma, hyperlipidemia, a recent diagnosis of anterior uveitis, and familial sarcoidosis presented with dyspnea, chest tightness, and worsening cough. Examination revealed anterior uveitis, erythema nodosum, jugular venous distension, and pedal edema. The electrocardiogram (ECG) demonstrated bifascicular block and premature ventricular contractions (PVCs). The brain natriuretic peptide (BNP) was 975 pg/mL, with the transthoracic echocardiogram revealing a left ventricular ejection fraction of 25-30% with global LV akinesis. Coronary computed tomography angiography (CCTA) excluded coronary artery disease. Cardiac MRI showed late gadolinium enhancement, with PET demonstrating active myocardial inflammation, supporting a >90% probability of CS. Given her clinical trajectory and risk of further decompensation, immunosuppressive therapy was initiated without pursuing a biopsy. A dual-chamber implantable cardioverter defibrillator (ICD) was placed due to risk of ventricular arrhythmias. Bronchoalveolar lavage (BAL) showed a CD4/CD8 ratio of 6.53, reinforcing the diagnosis. She responded well to treatment, with symptom improvement and repeat imaging demonstrating signs of disease remission. Conclusions: This case underscores the growing role of multimodal imaging in CS diagnosis, potentially replacing biopsy in select cases. Early imaging-based diagnosis enabled timely immunosuppression and ICD placement, improving outcomes.
Keywords: cardiac sarcoidosis; case report; endomyocardial biopsy; heart failure; multimodality imaging; non-invasive diagnosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Multimodality imaging features of systemic amyloidosis: a case report.BMC Cardiovasc Disord. 2025 Jan 2;25(1):1. doi: 10.1186/s12872-024-04441-6. BMC Cardiovasc Disord. 2025. PMID: 39748274 Free PMC article.
-
Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure: systematic review and economic evaluation.Health Technol Assess. 2014 Aug;18(56):1-560. doi: 10.3310/hta18560. Health Technol Assess. 2014. PMID: 25169727 Free PMC article.
-
Monomorphic sustained right ventricular outflow tract tachycardia in pregnancy with favorable outcomes: a case report.J Med Case Rep. 2025 Jul 15;19(1):346. doi: 10.1186/s13256-025-05387-9. J Med Case Rep. 2025. PMID: 40665437 Free PMC article.
-
A Fatal Case of Cardiac Sarcoidosis Presenting as Refractory Ascites.Cureus. 2025 Jun 10;17(6):e85718. doi: 10.7759/cureus.85718. eCollection 2025 Jun. Cureus. 2025. PMID: 40642681 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Terasaki F., Kusano K., Nakajima T., Yazaki Y., Morimoto S.-I., Culver D.A., Isobe M. The characteristics of Japanese guidelines on diagnosis and treatment of cardiac sarcoidosis compared with the previous guidelines. Sarcoidosis Vasc. Diffuse Lung Dis. 2022;39:e2022028. doi: 10.36141/svdld.v39i3.12531. - DOI - PMC - PubMed
-
- Hussain K., Shetty M. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2024. Cardiac Sarcoidosis. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
