Suspected Hematuria: Adverse Effects of Rivaroxaban in Older Adult Treated for Atrial Fibrillation
- PMID: 40729260
- PMCID: PMC12225455
- DOI: 10.3390/reports7010011
Suspected Hematuria: Adverse Effects of Rivaroxaban in Older Adult Treated for Atrial Fibrillation
Abstract
Background: The modern concept of pharmaceutical healthcare implies monitoring the pharmacotherapy outcomes and reporting adverse drug reactions.
Objective: To present a suspected hematuria as the adverse rivaroxaban reaction in a patient with atrial fibrillation observed by pharmacists in a community pharmacy.
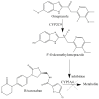
Case presentation: A 69-year-old female patient came to a pharmacy with a prescription for cranberry-based supplement. She was diagnosed with a mild urinary infection after experiencing blood in her urine for about two weeks. The pharmaceutical anamnesis revealed that the patient was treated with irbesartan and rivaroxaban. Rivaroxaban was applied for atrial fibrillation, and the patient was treated for nine months. The patient was treated with omeprazole gastro-resistant capsules for mild dyspepsia and stomach ache over a three-week period. The pharmacist counselled the patient to contact the clinician who introduced rivaroxaban, further suggesting substitution with different anticoagulant. Although the urine culture was negative, the physician introduced ciprofloxacin, which was followed by blood in the patient's stool. Thus, gastroscopy, colonoscopy, and gynecological examination were advised. All findings were normal. Four days after rivaroxaban was substituted with acenocoumarol, no blood in the urine or stool was detected.
Conclusions: Rivaroxaban can cause spot urine blood even when applied in therapeutic doses among older female patients when applied with omeprazole. Possible rivaroxaban interaction with omeprazole metabolites is suspected and should be carefully monitored.
Keywords: adverse drug reaction; ciprofloxacin; omeprazole; pharmaceutical healthcare; rivaroxaban.
Conflict of interest statement
Author Aleksandra Rapaić was employed by the pharmacy AU Biofarm. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation.Cochrane Database Syst Rev. 2013 Aug 8;(8):CD008980. doi: 10.1002/14651858.CD008980.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2018 Mar 06;3:CD008980. doi: 10.1002/14651858.CD008980.pub3. PMID: 23925867 Updated.
References
-
- World Health Organization (WHO) WHO Launches Global Effort to Halve Medication-Related Errors in 5 Years. [(accessed on 12 December 2023)]. Available online: https://www.who.int/news/item/29-03-2017-who-launches-global-effort-to-h....
Publication types
LinkOut - more resources
Full Text Sources