Tailoring oral targeted therapies dosage in lung cancer: A systematic review of pharmacokinetics studies on renal and hepatic impairment
- PMID: 40729346
- PMCID: PMC12306784
- DOI: 10.1371/journal.pone.0324056
Tailoring oral targeted therapies dosage in lung cancer: A systematic review of pharmacokinetics studies on renal and hepatic impairment
Abstract
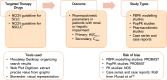
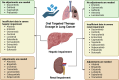
Background: Lung cancer is the leading cause of cancer-related deaths worldwide, and stage IV lung cancer is frequently managed with targeted therapy. Renal and hepatic impairment frequently coexist with cancer, often requiring a reduction in targeted therapy dosage. This systematic review assesses the appropriateness of current targeted therapy dosage adjustments in individuals with hepatic and renal impairment by comparing package insert recommendations with available pharmacokinetic studies.
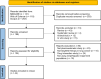
Methods: We reviewed the most recent guidelines from the National Comprehensive Cancer Network (NCCN) on the use of non-monoclonal antibody targeted therapy. We also examined all package inserts for information on dose adjustment in cases of hepatic and renal impairment. We then systematically searched for studies that involved pharmacokinetic analysis in populations with hepatic or renal impairment, as well as those undergoing hemodialysis and peritoneal dialysis.
Results: We identified 44 studies from 21 oral lung cancer therapies that met the inclusion criteria. We developed 13 new recommendations and updated 7 existing ones regarding targeted therapy dose adjustment in cases of hepatic and renal impairment compared to the information provided in the package insert. Several drugs have not published their pharmacokinetic results in a scientific journal, which limits access to their appropriateness. Moreover, there is a lack of research on pharmacokinetic analysis of targeted therapy in patients undergoing hemodialysis and peritoneal dialysis.
Conclusions: Adjusting the dosage of targeted therapy in hepatic and renal impairment based on pharmacokinetic analysis is essential to broaden the usage, improve effectiveness, and minimize side effects. Further pharmacokinetic research on the usage in unstudied populations is strongly advised.
Prospero registration number: CRD42024518123.
Copyright: © 2025 Hardi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews.Cochrane Database Syst Rev. 2014 Apr 29;2014(4):CD007768. doi: 10.1002/14651858.CD007768.pub3. Cochrane Database Syst Rev. 2014. PMID: 24777444 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

