Mapping cerebral blood perfusion and its links to multi-scale brain organization across the human lifespan
- PMID: 40729400
- PMCID: PMC12324687
- DOI: 10.1371/journal.pbio.3003277
Mapping cerebral blood perfusion and its links to multi-scale brain organization across the human lifespan
Abstract
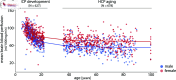
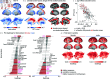
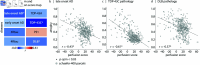
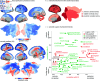
Blood perfusion delivers oxygen and nutrients to all cells, making it a fundamental feature of brain organization. How cerebral blood perfusion maps onto micro-, meso- and macro-scale brain structure and function is therefore a key question in neuroscience. Here we analyze pseudo-continuous arterial spin labeling (ASL) data from 1305 healthy individuals in the HCP Lifespan studies (5-22 and 36-100 years) to reconstruct a high-resolution normative cerebral blood perfusion map. At the cellular and molecular level, cerebral blood perfusion co-localizes with granular layer IV, biological pathways for maintenance of cellular relaxation potential and mitochondrial organization, and with neurotransmitter and neuropeptide receptors involved in vasomodulation. At the regional level, blood perfusion aligns with cortical arealization and is greatest in regions with high metabolic demand and resting-state functional hubs. Looking across individuals, blood perfusion is dynamic throughout the lifespan, follows micro-architectural changes in development, and maps onto individual differences in physiological changes in aging. In addition, we find that cortical atrophy in multiple neurodegenerative diseases (late-onset Alzheimer's disease, TDP-43C, and dementia with Lewy bodies) is most pronounced in regions with lower perfusion, highlighting the utility of perfusion topography as an indicator of transdiagnostic vulnerability. Finally, we show that ASL-derived perfusion can be used to delineate arterial territories in a data-driven manner, providing insights into how the vascular system is linked to human brain function. Collectively, this work highlights how cerebral blood perfusion is central to, and interlinked with, multiple structural and functional systems in the brain.
Copyright: © 2025 Farahani et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Presigny C, De Vico Fallani F. Colloquium: multiscale modeling of brain network organization. Rev Mod Phys. 2022;94(3). doi: 10.1103/revmodphys.94.031002 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

