A Prion-Like Domain in EBV EBNA1 Promotes Phase Separation and Enables SRRM1 Splicing
- PMID: 40729735
- PMCID: PMC12591112
- DOI: 10.1002/advs.202501977
A Prion-Like Domain in EBV EBNA1 Promotes Phase Separation and Enables SRRM1 Splicing
Abstract
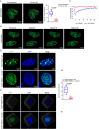
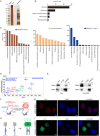
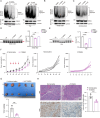
Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1) is necessary to maintain stability of EBV episomes, EBV replication, and causes host genomic instability and promotes tumor cells survival. Recent studies have shown that viruses utilize liquid-liquid phase separation (LLPS) within host cells to form sub-cellular compartments known as "virus factories". Prion-like domains (PrLDs), which resemble structural domains of low complexity, are shown to drive LLPS in vivo. In the current study, a PrLD is identified in EBNA1 and aggregation of EBNA1 proteins is observed in EBV-positive tumors. EBNA1 condensate interacting molecules are examined and are found that EBNA1 interacts with the splicing factor SRSF1 to regulate alternative splicing of SRRM1 and promote tumor progression. Deleting the EBNA1 PrLD results in defects in protein aggregation, LLPS, alternative splicing regulation, and nasopharyngeal carcinoma cells proliferation. Targeting the PrLD of EBNA1 inhibits the formation of protein aggregation, promotes alternative splicing of SRRM1, and inhibits the progression of nasopharyngeal carcinoma. Here, we report for the first time that EBNA1, a protein from the human oncogenic virus EBV, is a prion-like protein, combining algorithm prediction and experimental validation. That implies a possible molecular pathogenic mechanism of EBNA1 in neurodegenerative diseases.
Keywords: EBV‐encoded nuclear antigen 1; alternative splicing; phase separation; prion‐like domain; protein aggregation.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Young L. S., Rickinson A. B., Nat. Rev. Cancer 2004, 4, 757. - PubMed
-
- Young L. S., Yap L. F., Murray P. G., Nat. Rev. Cancer 2016, 16, 789. - PubMed
-
- Dunmire S. K., Verghese P. S., Balfour H. H. Jr., J. Clin Virol. 2018, 102, 84. - PubMed
-
- Hussein K., Rath B., Ludewig B., Kreipe H., Jonigk D., Eur. J. Cancer 2014, 50, 2417. - PubMed
MeSH terms
Substances
Grants and funding
- 111-2-12/China 111 Project
- 23A0016/Department of Education of Hunan Province
- kq2502216/Natural Science Foundation of Changsha
- GZC20242037/Postdoctoral Fellowship Program of CPSF
- 140050041/Postdoctoral Science Foundation of Central South University
- 2024M753677/China Postdoctoral Science Foundation
- 2025M771479/China Postdoctoral Science Foundation
- 2024JJ4025/Science and Technology Program of Hunan Province
- 2024RC3231/Science and Technology Program of Hunan Province
- 2024JJ6551/Science and Technology Program of Hunan Province
- 2025JK2138/Science and Technology Program of Hunan Province
- 82472701/National Natural Science Foundation of China
- 82203233/National Natural Science Foundation of China
- 32000665/National Natural Science Foundation of China
- 82060042/National Natural Science Foundation of China
- 82373062/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
