Corticosterone stimulates synthesis of 2-arachidonoylglycerol via putative membrane-bound glucocorticoid receptors and inhibits GABA release via CB1 cannabinoid receptors in the ventrolateral periaqueductal gray
- PMID: 40729946
- PMCID: PMC12402929
- DOI: 10.1016/j.molpha.2025.100058
Corticosterone stimulates synthesis of 2-arachidonoylglycerol via putative membrane-bound glucocorticoid receptors and inhibits GABA release via CB1 cannabinoid receptors in the ventrolateral periaqueductal gray
Abstract
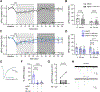
The ventrolateral periaqueductal gray (vlPAG) plays a critical role in pain modulation. GABAergic neurotransmission within the vlPAG regulates the descending pain pathway. This study investigates the mechanisms through which corticosterone (CORT) modulates GABA release in the vlPAG via putative membrane-associated glucocorticoid receptors (mbGRs). Superfusion of CORT decreases evoked inhibitory postsynaptic currents in a mbGR- and CB1 cannabinoid receptor (CB1R)-dependent manner. Using a depolarization-induced suppression of inhibition protocol to test the effects of CORT on the endocannabinoid system, we find that CORT-mediated signaling enhances 2-arachidonoylglycerol synthesis that is inhibited by the diacylglycerol lipase inhibitor, DO34. CORT prolongs CB1R activation through a Gαs and protein kinase A-dependent pathway, whereas early depolarization-induced suppression of inhibition-initiated endocannabinoid activation of CB1Rs is independent of protein kinase A. These results highlight the critical role of CORT in the vlPAG in engaging endocannabinoid pathways to inhibit GABA release. The results indicate that CORT activation of putative mbGRs promote activation of the descending pain modulatory pathway through CB1R-mediated inhibition of GABA release in the vlPAG. SIGNIFICANCE STATEMENT: This study provides evidence that corticosterone activates putative membrane glucocorticoid receptors to increase levels of 2-arachidonoylglycerol to activate presynaptic CB1 cannabinoid receptors. These findings reveal mechanisms by which stress modulates the ventrolateral periaqueductal gray and the descending pain circuit.
Keywords: Corticosterone; Diacylglycerol lipase; Endocannabinoid; Periaqueductal gray; Protein kinase A.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest.
Figures
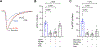

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

