Effects of Semaglutide With or Without Concomitant Mineralocorticoid Receptor Antagonist Use in Participants With Type 2 Diabetes and Chronic Kidney Disease: A FLOW Trial Prespecified Secondary Analysis
- PMID: 40730031
- PMCID: PMC12583412
- DOI: 10.2337/dc25-0472
Effects of Semaglutide With or Without Concomitant Mineralocorticoid Receptor Antagonist Use in Participants With Type 2 Diabetes and Chronic Kidney Disease: A FLOW Trial Prespecified Secondary Analysis
Abstract
Objective: In the Evaluate Renal Function With Semaglutide Once Weekly (FLOW) trial, semaglutide reduced the risk of major kidney and cardiovascular (CV) outcomes and all-cause mortality in people with type 2 diabetes (T2D) and chronic kidney disease (CKD). This prespecified analysis assessed the effects of semaglutide on kidney, CV, and mortality outcomes by baseline mineralocorticoid receptor antagonist (MRA) use.
Research design and methods: Participants were randomized to once-weekly subcutaneous semaglutide 1.0 mg or placebo. The primary kidney outcome was a composite of time to first persistent ≥50% eGFR reduction from baseline, kidney failure, or death from kidney/CV causes. Baseline MRA was predominantly spironolactone; finerenone was only available after recruitment ended.
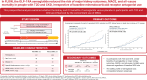
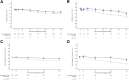
Results: Effects were analyzed by baseline MRA use (n = 257 [136 in the semaglutide group and 121 in the placebo group]) and nonuse (n = 3,276 [1,631 in the semaglutide group and 1,645 in the placebo group]). Semaglutide reduced the risk of the primary kidney outcome by 49% (59 events; hazard ratio [HR] 0.51 [95% CI 0.30, 0.86]) and 21% (682 events; HR 0.79 [95% CI 0.68, 0.92]; P-interaction = 0.12) versus placebo in MRA and non-MRA subgroups, respectively. There was no heterogeneity, favoring the effects of semaglutide on major adverse CV events (MACE) and all-cause mortality in both MRA subgroups (P-interaction > 0.7). Albuminuria at 104 weeks was reduced from baseline with semaglutide by 15% (95% CI -41, 31) in MRA users and 33% (26, 39) in nonusers versus placebo (P-interaction = 0.22). Estimated glomerular filtration rate decline was similarly reduced with semaglutide (P-interaction = 0.71). The safety profile of semaglutide was comparable between subgroups.
Conclusions: In participants with T2D and CKD, consistent benefits of semaglutide on major kidney outcomes, MACE, and all-cause mortality were observed regardless of baseline MRA use.
© 2025 by the American Diabetes Association.
Figures


Comment in
-
The FLOW of Progress in Diabetic Kidney Disease: Semaglutide's Role in Combination Therapy.Diabetes Care. 2025 Nov 1;48(11):1875-1877. doi: 10.2337/dci25-0088. Diabetes Care. 2025. PMID: 41115233 No abstract available.
References
-
- International Diabetes Federation . IDF Diabetes Atlas, 10th Edition. 2021. Accessed 28 February 2023. Available from https://diabetesatlas.org
-
- Wen CP, Chang CH, Tsai MK, et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int 2017;92:388–396 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

