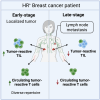
Tumor-specific CD8 T cell characterization in HR+ breast cancer reveals an impaired antitumoral response in patients with lymph node metastasis
- PMID: 40730190
- PMCID: PMC12432377
- DOI: 10.1016/j.xcrm.2025.102252
Tumor-specific CD8 T cell characterization in HR+ breast cancer reveals an impaired antitumoral response in patients with lymph node metastasis
Abstract
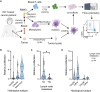
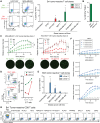
Most breast cancers express the estrogen receptor (ER), but the immune response of hormone receptor-positive (HR+) breast cancer remains poorly characterized. Here, dendritic cells loaded with tumor lysate are used to identify tumor-reactive CD8 T cells, which are detected in most HR+ breast cancer patients, especially those with early-stage tumors. When present, the circulating antitumor CD8 response contains cytotoxic T cells with diverse specificity and T cell receptor (TCR) repertoire. Additionally, patients with blood cancer-specific T cells have significantly more CD8 tumor-infiltrating lymphocytes (TILs). Moreover, tumor-reactive TCR sequences are detected in the tumor, but at a significantly lower proportion in patients with lymph node involvement. Our data suggest that HR+ breast cancer patients with lymph node metastasis lack tumor-specific CD8 T cells with capacity to infiltrate the tumor at significant levels. However, early-stage patients have a diverse antitumor CD8 response that could be harnessed to develop immunotherapeutic approaches for late-stage HR+ patients.
Keywords: CDR3; CTA; ER+ breast cancer; T cell receptor; T cell repertoire; antitumor CD8 T cells; cancer testis antigen; metastatic luminal breast cancer; moDC; tumor lysate; tumor-specific T cells.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

