Enhancing adoptive cell therapy: future strategies for immune cell radioprotection in neuro-oncology
- PMID: 40730675
- PMCID: PMC12307653
- DOI: 10.1038/s41698-025-01059-5
Enhancing adoptive cell therapy: future strategies for immune cell radioprotection in neuro-oncology
Abstract
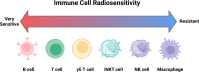
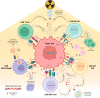
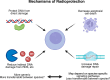
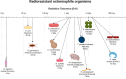
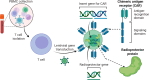
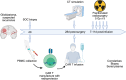
Adoptive cell therapy (ACT), particularly chimeric antigen receptor T cell (CAR T) therapy, has emerged as a promising approach in cancer treatment, demonstrating efficacy in hematological malignancies but facing challenges in brain tumors. The combination of ACT with radiation therapy (RT) offers a potential strategy to enhance therapeutic outcomes, as RT can stimulate immune responses by promoting antigen presentation and T cell recruitment. However, a major hurdle is the radiosensitivity of immune cells, leading to their rapid depletion within the radiation field, which undermines the benefits of this combination. This review explores strategies to increase the radioresistance of immune cells, highlighting the need for innovative radioprotective approaches. We discuss the potential of extremophile-derived molecules, such as the Damage Suppressor protein from tardigrades, as novel radioprotectants that could be integrated into ACT protocols. Furthermore, we address key considerations for clinical trial design, including the sequencing of RT and ACT, dosing parameters, and safety considerations. By bridging insights from extremophile biology and immuno-oncology, this work aims to optimize the efficacy of ACT in the challenging context of brain tumors, paving the way for enhanced treatment strategies in neuro-oncology.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Z.J.R. is listed as an inventor on intellectual property related to brain tumor diagnostics that is managed by Duke and has been licensed to Genetron Health. The Authors declare no competing Non-Financial Interests.
Figures



Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
B cell antigens: A key to optimizing CAR-T cell therapy.Int Rev Immunol. 2025 Jun 19:1-28. doi: 10.1080/08830185.2025.2515839. Online ahead of print. Int Rev Immunol. 2025. PMID: 40537997 Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Efficient combination of radiotherapy and CAR-T - A systematic review.Biomed Pharmacother. 2024 May;174:116532. doi: 10.1016/j.biopha.2024.116532. Epub 2024 Apr 3. Biomed Pharmacother. 2024. PMID: 38574625
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
References
-
- Albelda, S. M. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat. Rev. Clin. Oncol.21, 47–66 (2024). - PubMed
-
- Mullard, A. FDA approves first tumour-infiltrating lymphocyte (TIL) therapy, bolstering hopes for cell therapies in solid cancers. Nat. Rev. Drug Discov.23, 238 (2024). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

