Safety and immunogenicity of fractional COVID-19 vaccine doses in Nigerian adults: A randomized non-inferiority trial
- PMID: 40730836
- PMCID: PMC12307607
- DOI: 10.1038/s41598-025-06536-2
Safety and immunogenicity of fractional COVID-19 vaccine doses in Nigerian adults: A randomized non-inferiority trial
Erratum in
-
Correction: Safety and immunogenicity of fractional COVID-19 vaccine doses in Nigerian adults: A randomized non-inferiority trial.Sci Rep. 2025 Aug 20;15(1):30530. doi: 10.1038/s41598-025-16245-5. Sci Rep. 2025. PMID: 40836008 Free PMC article. No abstract available.
Abstract
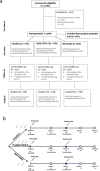
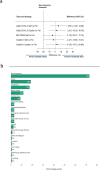
Fractional dosing of vaccines is a viable strategy to extend COVID-19 vaccine supplies in resource-constrained settings. We did a triple-blinded, multi-site, randomized non-inferiority trial in Nigeria (PACTR202206754734018). Adults 18-65 years received full, half, or quarter primary doses of ChAdOx1 or Ad26.COV2.S, or full vs half doses of BNT162b2. Primary study outcome was seroconversion defined as ≥ 2.5-fold rise in anti-Spike IgG geometric-mean fold rise (GMFR) at day 28. A total of 1894 participants were enrolled between June 21, 2022, and January 25, 2023. 320 participants in the fractional dose group and 220 in the standard dose group completed follow-up and were included in the analysis. Seropositivity at baseline was high, at 68% (365/539). Seroconversion was comparable between standard and fractional doses (p = 0.822). For ChAdOx1, 31% achieved seroconversion at standard dose (16/52), 28% at half-dose (15/53), and 34% in quarter-dose (18/53). For Ad26.COV2.S, the proportions were 27% (28/105), 32% (22/68), and 30% (21/71) respectively. For BNT162b2, the proportions were 43% (27/63) and 39% (29/75) for standard- and half-dose. Serum neutralization showed ≥ twofold response across dosing. There were no serious adverse events. Fractional vaccine doses generated non-inferior immune responses compared to standard doses in the context of previous COVID-19.Protocol Registration: The protocol was registered with the Pan African Clinical Trials Registry (PACTR) PACTR202206754734018.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: All procedures performed in studies involving human participants were following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent: Ethical approval was obtained from the National Health Research and Ethics Committee, the Institutional Review Board (IRB) of NIMR, Lagos, and the ethics committee of the trial institution. The purpose, processes, and expected outcome of the study were explained to all participants and/or their caregivers and informed consent was obtained before the commencement of the study. Confidentiality was maintained, and the freedom to withdraw at any time from the study without negative consequences was emphasized.
Figures




References
-
- World Health Organization. COVID-19 Vaccines. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19.... (2022)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

