Single urinary extracellular vesicle proteomics identifies complement receptor CD35 as a biomarker for sepsis-associated acute kidney injury
- PMID: 40730843
- PMCID: PMC12307914
- DOI: 10.1038/s41467-025-62229-4
Single urinary extracellular vesicle proteomics identifies complement receptor CD35 as a biomarker for sepsis-associated acute kidney injury
Abstract
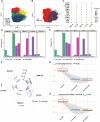
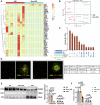
Sepsis-associated acute kidney injury (SA-AKI) portends severe health burden due to significant morbidity and mortality, while early diagnosis remains challenging. In this study, proximity-dependent barcoding assay (PBA) is established to profile the surface proteome of single urinary extracellular vesicle (uEV). Principle uEV clusters with unique function and origination are profiled in SA-AKI in a screening cohort. Complement receptor CD35 on single uEV (CD35-uEV) displays high diagnostic accuracy for SA-AKI (AUC-ROC 0.89 in validation cohort, n = 134). Besides, CD35-uEV enables identification of subclinical AKI (AUC-ROC 0.84 in prospective cohort, n = 72). Moreover, CD35-uEV correlates closely with AKI severity which also predicts persistent AKI (AUC-ROC 0.77), mortality risks (AUC-ROC 0.70) and progression to AKD (AUC-ROC 0.66). Multi-omics profiling reveals that CD35-uEV are predominantly released from injured podocytes exhibiting diminished CD35 expression. Overall, this study identifies a single uEV biomarker related to injured podocyte for early diagnosis and risk stratification of SA-AKI.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Balkrishna, A. et al. Sepsis-mediated renal dysfunction: pathophysiology, biomarkers and role of phytoconstituents in its management. Biomed. Pharmacother.165, 115183 (2023). - PubMed
-
- van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol.19, 213–228 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

