High-risk human papillomavirus testing for underscreened populations: cost-effectiveness and affordability in three country settings
- PMID: 40731006
- PMCID: PMC12305891
- DOI: 10.1186/s12889-025-23791-0
High-risk human papillomavirus testing for underscreened populations: cost-effectiveness and affordability in three country settings
Abstract
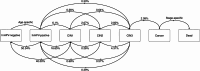
Background: The high-risk human papillomavirus (hrHPV)-based screening recommended by the World Health Organization is expected to lead to worldwide reduction of the cervical cancer burden, but the countries burdened most by cervical cancer also struggle with the costs of transitioning to this approach. Country-specific evaluations are needed to inform policymakers on implementation of hrHPV-based screening for their setting. Following initial implementation in Uganda, Bangladesh and Slovakia focused on underscreened women in the PRESCRIP-TEC project, we investigated the potential cost-effectiveness and affordability of hrHPV-based screening strategies.
Methods: Country-specific model-based cost-effectiveness and budget impact analyses were conducted for the three countries, comparing the PRESCRIP-TEC strategy with the existing screening strategy in each setting. Data from initial project implementation informed the relevant model parameters.
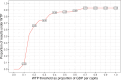
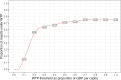
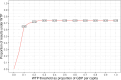
Results: The PRESCRIP-TEC strategy resulted in disability-adjusted life year (DALY) gains in all three countries. The cervical cancer incidence rate was reduced by a third for Uganda, 15% for Bangladesh and 11% for Slovakia. The incremental cost-effectiveness ratios were UGX 0.56 million per DALY for Uganda (I$ 475), BDT 76 thousand per DALY for Bangladesh (I$ 1698) and EUR 1782 (I$ 3637) per DALY for Slovakia. Substantial additional funding will be required to enable implementation, particularly in relation to the initial start-up costs.
Conclusions: The provided estimates can serve to inform policymakers and researchers in the context of implementing hrHPV-based screening in diverse settings.
Keywords: Budget impact; Cervical cancer; Cost-effectiveness; Economic evaluation; High-risk human papillomavirus; Screening.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
-
- Descamps P, Dixon S, Bosch Jose FX, Kyrgiou M, Monsonego J, Neisingh O, et al. Turning the tide—Recommendations to increase cervical cancer screening among women who are underscreened. Int J Gynecol Obstet. 2024;166:3–21. - PubMed

