Proline dehydrogenase, a rate-limiting catabolic enzyme, affecting the growth and pathogenicity of Toxoplasma gondii tachyzoites by regulating the proline metabolism and mitochondrial function of the parasite
- PMID: 40731026
- PMCID: PMC12308898
- DOI: 10.1186/s13071-025-06966-x
Proline dehydrogenase, a rate-limiting catabolic enzyme, affecting the growth and pathogenicity of Toxoplasma gondii tachyzoites by regulating the proline metabolism and mitochondrial function of the parasite
Abstract
Background: The pathogenicity of Toxoplasma gondii is closely associated with its intracellular lytic cycle in host cells. Currently, the mechanisms by which T. gondii completes the lytic cycle remain unclear. The proline metabolism has been reported to be crucial for intracellular growth of pathogens by providing energy and nutrients. However, it remains unclear whether the intracellular growth and pathogenicity of T. gondii are related to proline metabolism.
Methods: The gene-edited strains of proline dehydrogenase (Tgprodh) were constructed by using clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR-Cas9) technology. The effects of the Tgprodh gene on the growth in vitro and pathogenicity in vivo of the tachyzoites for T. gondii were studied through proliferation, plaque, invasion, egress and virulence assays. The effects of the Tgprodh gene on mitochondrial function were studied by using reactive oxygen species (ROS), mitochondrial membrane potential (∆Ψm), adenosine triphosphate (ATP) assay kits, mitochondrial DNA (mtDNA) copy numbers, transmission electron microscopy (TEM) analysis, and reverse transcriptase quantitative polymerase chain reaction (RT-qPCR). The effects of the Tgprodh gene on proline metabolism were studied by using L-proline (L-Pro), L-glutamic acid (L-Glu), L-glutamine (L-Gln) assay kits, and RT-qPCR.
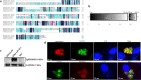
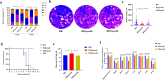
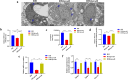
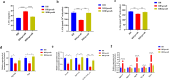
Results: TgPRODH, the first rate-limiting enzyme in proline metabolism, was identified to be encoded by T. gondii and localized in the cytoplasm of T. gondii. Deletion of the Tgprodh gene resulted in significant growth inhibition in vitro and reduced pathogenicity in vivo of T. gondii. Further study found that deletion of the Tgprodh gene caused damage to the mitochondrial morphology, decreased membrane potential, mtDNA copy numbers, and the production of ATP and ROS. The expression of genes for maintaining mitochondrial integrity was downregulated in the Tgprodh-knockout strain of T. gondii, while complementation of the Tgprodh gene restored these defects in this parasite. Meantime, the deletion of the Tgprodh gene resulted in the accumulation of proline, reduced the contents of glutamate and glutamine, and affected the expression of genes related to proline catabolism in T. gondii.
Conclusions: The present study found the significance of the Tgprodh gene for the intracellular growth and pathogenicity of T. gondii through regulating mitochondrial function and the proline metabolism and provided a novel insight to reveal intracellular survival strategies of T. gondii.
Keywords: Tgprodh; Toxoplasma gondii; Growth; Mitochondrial function; Proline metabolism.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were performed in accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China. The protocol of the animal assay was reviewed and approved by the Institutional Animal Care and Use Committee of Northwest A&F University. All mice were euthanized with sodium pentobarbital after the experiment ended. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
[Generation of a dense granule protein 3 gene-deficient strain of Toxoplasma gondii and its virulence testing].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2025 Jun 4;37(3):304-309. doi: 10.16250/j.32.1915.2024293. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2025. PMID: 40730529 Chinese.
-
Elucidating the Role of Toxoplasma gondii's Mitochondrial Superoxide Dismutase.Biomolecules. 2025 Jul 7;15(7):972. doi: 10.3390/biom15070972. Biomolecules. 2025. PMID: 40723844 Free PMC article.
-
The apicoplast localized isocitrate dehydrogenase is needed for de novo fatty acid synthesis in the apicoplast of Toxoplasma gondii.Front Cell Infect Microbiol. 2025 Jun 24;15:1542122. doi: 10.3389/fcimb.2025.1542122. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40630641 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

