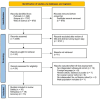
The role of ultrasound-guided tru-cut biopsy in ovarian cancer: a systematic review of its safety, adequacy, and accuracy with meta-analysis of diagnostic performance
- PMID: 40731027
- PMCID: PMC12308927
- DOI: 10.1186/s13048-025-01739-7
The role of ultrasound-guided tru-cut biopsy in ovarian cancer: a systematic review of its safety, adequacy, and accuracy with meta-analysis of diagnostic performance
Abstract
Objectives: To analyze the safety, adequacy and accuracy of ultrasound-guided tru-cut biopsy in the diagnosis of ovarian cancer.
Methods: A systematic search of PubMed, Web of Science, and Scopus was conducted through June 2024. Studies meeting predefined criteria were included in the review. The quality of diagnostic accuracy studies was assessed using QUADAS-2. A meta-analysis was performed on studies reporting complete 2 × 2 diagnostic data.
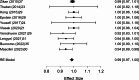
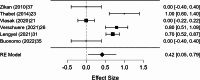
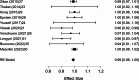
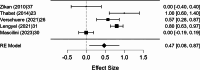
Results: A total of 2,250 articles were initially screened, and after the removal of duplicates, 54 articles were deemed eligible for full-text assessment. Ultimately, 18 studies, comprising 1,867 patients who underwent ultrasound-guided tru-cut biopsy, were included in the systematic review. A total of 16 complications were reported across 1,898 biopsies performed in the included studies, resulting in a mean complication rate of 0.58% (95% CI: 0.187- 0.964%). Adequacy for histological and immunohistochemical examination after one attempt was reported in 16 studies, with a mean adequacy rate of 95.1% (95% CI: 92.69- 97.50%) and a median rate of 95.97%. Diagnostic accuracy was assessed in 13 studies, revealing a mean diagnostic accuracy of 95.54% (95% CI: 93.19- 97.89%) and a median of 97.48%.In the meta-analysis of 10 studies, pooled sensitivity was 98.6%, specificity 41.9%, positive predictive value (PPV) 99.0%, and negative predictive value (NPV) 47.2%, with high heterogeneity observed in specificity and NPV estimates.
Conclusions: Ultrasound guided tru-cut biopsy is a safe and effective diagnostic method, demonstrating a high adequacy rate for histological and immunohistochemical analysis. It shows excellent performance in confirming malignancy and supports preoperative decision making. To further define its role in the diagnostic pathway for ovarian cancer, additional prospective multicenter studies are needed-both to validate its reliability in negative cases and to ensure tissue adequacy for advanced molecular testing in the context of personalized medicine.
Keywords: Core needle biopsy; Ovarian cancer; Systematic review; Tru-cut biopsy; Ultrasound.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2021;71(3):209–249. 10.3322/caac.21660 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical