Comparative analysis of 3D-culture techniques for multicellular colorectal tumour spheroids and development of a novel SW48 3D-model
- PMID: 40731083
- PMCID: PMC12307937
- DOI: 10.1038/s41598-025-13588-x
Comparative analysis of 3D-culture techniques for multicellular colorectal tumour spheroids and development of a novel SW48 3D-model
Abstract
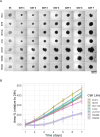
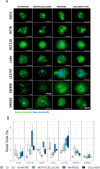
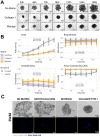
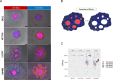
Colorectal cancer (CRC) is an important global health challenge, with nearly 2 million diagnosed cases and over 900,000 deaths annually despite therapeutic advancements. The high morbidity and mortality rates underscore the need for more efficient therapies. Three-dimensional (3D) cell culture models have emerged as more physiologically relevant alternatives to traditional two-dimensional (2D) models for drug screening and mechanistic studies. However, generating consistent spheroids across different CRC cell lines presents technical challenges, and protocols remain inconsistent. This study evaluated different 3D culture methodologies, i.e. overlay on agarose, hanging drop, and U-bottom plates without matrix or with methylcellulose, Matrigel or collagen type I hydrogels, across eight CRC cell lines. Multicellular tumour spheroids (MCTS) morphology and cell viability were analysed. Co-cultures with immortalised colonic fibroblasts were explored to improve the physiological relevance of the tumour models. The study provided insights into the morphological and viability characteristics of 3D cultures across multiple CRC cell lines. A novel compact spheroid model using the SW48 cell line was successfully developed. Co-culture experiments with fibroblasts offered additional insights into tumour-stroma interactions in a 3D setting. This study contributes to the advancement of more physiologically relevant in vitro CRC models, potentially enhancing the accuracy of preclinical studies and drug screening processes. The successful 3D model of SW48 expands the repertoire of CRC cell lines available for 3D culture studies. The treatment of regular multi-well plates with anti-adherence solution allows to generate CRC spheroids at significantly lower cost than using cell-repellent multi-well plates. These findings may lead to improved preclinical models for CRC research and drug development.
Keywords: Colorectal cancer 3D models; Tumour spheroids.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: All authors have read and agreed to the published version of the manuscript. Competing interests: The authors declare no competing interests.
Figures



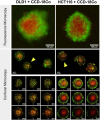
References
-
- Morgan, E. et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut72, 338–344 (2023). - PubMed
-
- Schmoll, H. J. et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol.23, 2479–2516 (2012). - PubMed
-
- Van Cutsem, E. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol.27, 1386–1422 (2016). - PubMed
-
- Araghi, M. et al. Colon and rectal cancer survival in seven high-income countries 2010–2014: variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut70, 114–126 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

