Whole exome sequencing study of adamantinomatous craniopharyngioma reveals the mutational characteristics of recurrent cases
- PMID: 40731085
- PMCID: PMC12367868
- DOI: 10.1007/s11060-025-05061-6
Whole exome sequencing study of adamantinomatous craniopharyngioma reveals the mutational characteristics of recurrent cases
Abstract
Purpose: Adamantinomatous craniopharyngioma (ACP) is a benign but surgically challenging intracranial tumor, and its high postoperative recurrence rate poses a threat to patient prognosis. In this study, whole exome sequencing (WES) was performed to comprehensively understand the genomic alterations in ACP.
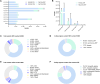
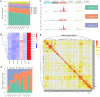
Methods: First, the gene mutation profile of ACP was acquired through an overview of the WES data. The somatic single nucleotide variants (SNV) mutational spectrum and signature of ACP were analyzed. Subsequently, somatic mutation data were used to identify the characteristic SNV of recurrent ACP to further explore the mechanism underlying recurrence, and the effect of these mutations on protein expression was verified through immunohistochemical and immunofluorescence analyses.
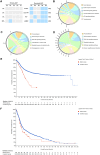
Results: We obtained the overall mutational landscape of ACP and identified four key mutated genes in recurrent samples: Procollagen-Lysine,2-Oxoglutarate 5-Dioxygenase 3 (PLOD3), Collagen Type XVII Alpha 1 chain (COL17A1), Fibronectin 1 (FN1), and Laminin Subunit Beta 3 (LAMB3). Among these, we verified that COL17A1, FN1, and LAMB3 expression was mainly located in the keratin nodule structures in tumors and was elevated in recurrent cases.
Conclusion: PLOD3, COL17A1, FN1, and LAMB3 were identified as the key mutated genes in recurrent samples. These findings could provide a new perspective for understanding the tumorigenesis and recurrence mechanisms of ACP. A deeper exploration is needed to determine whether these key mutated genes promote ACP recurrence and drive the overproduction of keratin nodules in recurrent cases.
Keywords: Adamantinomatous craniopharyngioma; Mutation; Recurrent tumor; Whole exome sequencing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study was approved by the Institutional Review Board of Sanbo Brain Hospital (Approval NO. SBNK-YJYS-2023-031-01). Informed consent was obtained from all individual participants included in the study. Consent to participate: Informed consent was obtained from all individual participants or their parent or legal guardian included in the study. Competing interests: The authors declare no competing interests.
Figures


References
-
- Muller HL (2014) Craniopharyngioma. Endocr Rev 35(3):513–543 - PubMed
-
- Nielsen EH et al (2011) Incidence of craniopharyngioma in Denmark (n = 189) and estimated world incidence of craniopharyngioma in children and adults. J Neurooncol 104(3):755–763 - PubMed
-
- Muller-Scholden J et al (2000) Radical surgery in a neonate with craniopharyngioma. Report of a case. Pediatr Neurosurg 33(5):265–269 - PubMed
-
- Kageji T et al (2017) Congenital craniopharyngioma treated by radical surgery: case report and review of the literature. Childs Nerv Syst 33(2):357–362 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

