Senescence-associated lysosomal dysfunction impairs cystine deprivation-induced lipid peroxidation and ferroptosis
- PMID: 40731111
- PMCID: PMC12307602
- DOI: 10.1038/s41467-025-61894-9
Senescence-associated lysosomal dysfunction impairs cystine deprivation-induced lipid peroxidation and ferroptosis
Abstract
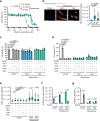
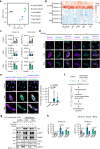
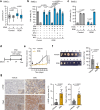
Senescent cells, characterized by irreversible cell cycle arrest and inflammatory factor secretion, promote various age-related pathologies. Senescent cells exhibit resistance to ferroptosis, a form of iron-dependent cell death; however, the underlying mechanisms remain unclear. Here, we discovered that lysosomal acidity was crucial for lipid peroxidation and ferroptosis induction by cystine deprivation. In senescent cells, lysosomal alkalinization causes the aberrant retention of ferrous iron in lysosomes, resulting in resistance to ferroptosis. Treatment with the V-ATPase activator EN6 restored lysosomal acidity and ferroptosis sensitivity in senescent cells. A similar ferroptosis resistance mechanism involving lysosomal alkalinization was observed in pancreatic cancer cell lines. EN6 treatment prevented pancreatic cancer development in xenograft and Kras mutant mouse models. Our findings reveal a link between lysosomal dysfunction and the regulation of ferroptosis, suggesting a therapeutic strategy for the treatment of age-related diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell179, 813–827 (2019). - PubMed
-
- Takahashi, A. et al. Mitogenic signalling and the p16INK4a–Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol.8, 1291–1297 (2006). - PubMed
-
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell186, 243–278 (2023). - PubMed
-
- Kim, Y., Jang, Y., Kim, M.-S. & Kang, C. Metabolic remodeling in cancer and senescence and its therapeutic implications. Trends Endocrinol. Metab.35, 732–744 (2024). - PubMed
-
- Ma, S. et al. Spatial transcriptomic landscape unveils immunoglobin-associated senescence as a hallmark of aging. Cell187, 7025–7044 (2024). - PubMed
MeSH terms
Substances
Grants and funding
- JPMJMS2217/MEXT | Japan Science and Technology Agency (JST)
- JPMJMS2022/MEXT | Japan Science and Technology Agency (JST)
- JPMJPF2202/MEXT | JST | Center of Innovation Program (COI)
- 24gm1710013h0001/Japan Agency for Medical Research and Development (AMED)
- 23K17420/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

