Developing and validating machine learning models to predict next-day extubation
- PMID: 40731125
- PMCID: PMC12307926
- DOI: 10.1038/s41598-025-12264-4
Developing and validating machine learning models to predict next-day extubation
Abstract
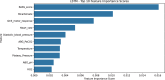
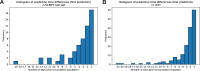
Criteria to identify patients who are ready to be liberated from mechanical ventilation (MV) are imprecise, often resulting in prolonged MV or reintubation, both of which are associated with adverse outcomes. Daily protocol-driven assessment of the need for MV leads to earlier extubation but requires dedicated personnel. We sought to determine whether machine learning (ML) applied to the electronic health record could predict next-day extubation. We examined 37 clinical features aggregated from 12AM-8AM on each patient-ICU-day from a single-center prospective cohort study of patients in our quaternary care medical ICU who received MV. We also tested our models on an external test set from a community hospital ICU in our health care system. We used three data encoding/imputation strategies and built XGBoost, LightGBM, logistic regression, LSTM, and RNN models to predict next-day extubation. We compared model predictions and actual events to examine how model-driven care might have differed from actual care. Our internal cohort included 448 patients and 3,095 ICU days, and our external test cohort had 333 patients and 2,835 ICU days. The best model (LSTM) predicted next-day extubation with an AUROC of 0.870 (95% CI 0.834-0.902) on the internal test cohort and 0.870 (95% CI 0.848-0.885) on the external test cohort. Across multiple model types, measures previously demonstrated to be important in determining readiness for extubation were found to be most informative, including plateau pressure and Richmond Agitation Sedation Scale (RASS) score. Our model often predicted patients to be stable for extubation in the days preceding their actual extubation, with 63.8% of predicted extubations occurring within three days of true extubation. Our findings suggest that an ML model may serve as a useful clinical decision support tool rather than complete replacement of clinical judgement. However, any ML-based model should be compared with protocol-based practice in a prospective, randomized controlled trial to determine improvement in outcomes while maintaining safety as well as cost effectiveness.
Keywords: Critical care; Deep learning; Machine learning; Mechanical ventilation; Respiratory failure.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: BDS holds US patent 10,905,706, “Compositions and methods to accelerate resolution of acute lung inflammation,” and serves on the scientific advisory board of Zoe Biosciences, in which he holds stock options. Other authors have no conflicts within the area of this work.
Figures


References
-
- Melsen, W. G. et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis.13(8), 665–671 (2013). - PubMed
-
- Epstein, S. K. & Ciubotaru, R. L. Independent effects of etiology of failure and time to reintubation on outcome for patients failing extubation. Am. J. Respir. Crit. Care Med.158(2), 489–493 (1998). - PubMed
-
- Ely, E. W. et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N. Engl. J. Med. New Engl. J. Med. (NEJM/MMS)35(25), 1864–1869 (1996). - PubMed
-
- Girard, T. D. et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): A randomised controlled trial. Lancet371(9607), 126–134 (2008). - PubMed
-
- Burns, K. E. A., Rizvi, L., Cook, D. J., Lebovic, G., Dodek, P., Villar, J., Slutsky, A. S., Jones, A., Kapadia, F. N., Gattas, D. J., Epstein, S. K., Pelosi, P., Kefala, K., Meade, M. O., Canadian Critical Care Trials Group. Ventilator weaning and discontinuation practices for critically ill patients. JAMA Am. Med. Assoc. (AMA)325(12), 1173–1184 (2021). - PMC - PubMed
MeSH terms
Grants and funding
- I01 CX001777/CX/CSRD VA/United States
- R01 HL153122/HL/NHLBI NIH HHS/United States
- U19 AI181102/AI/NIAID NIH HHS/United States
- R21 AG075423/AG/NIA NIH HHS/United States
- K23 HL169815/HL/NHLBI NIH HHS/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- U19 AI135964/AI/NIAID NIH HHS/United States
- R01 HL153312/HL/NHLBI NIH HHS/United States
- R01 HL158139/HL/NHLBI NIH HHS/United States
- P01 HL154998/HL/NHLBI NIH HHS/United States
- R01 LM013337/LM/NLM NIH HHS/United States
- R01 HL154686/HL/NHLBI NIH HHS/United States
- R01 HL147575/HL/NHLBI NIH HHS/United States
- R01 HL149883/HL/NHLBI NIH HHS/United States
- P01 AG049665/AG/NIA NIH HHS/United States
- 24PRE1196998/AHA/American Heart Association-American Stroke Association/United States
- U01 TR003528/TR/NCATS NIH HHS/United States
- U19AI135964/National Institute of Allergy and Infectious Diseases
LinkOut - more resources
Full Text Sources

