Place of dexamethasone implant as an adjunctive treatment in Coats' disease: a retrospective single-center study
- PMID: 40731271
- PMCID: PMC12309173
- DOI: 10.1186/s12886-025-04279-2
Place of dexamethasone implant as an adjunctive treatment in Coats' disease: a retrospective single-center study
Abstract
Background: To evaluate the efficacy and safety of intravitreal dexamethasone implant as an adjunctive treatment to ablative therapy in Coats' disease.
Methods: Consecutive Coats patients examined between February 2012 and January 2024 who received Dexamethasone implant with a follow-up for over twelve months were retrospectively evaluated. The demographic assessment, clinical staging, therapies, and clinical and anatomic outcomes were noted.
Results: Of 15 total patients, four were excluded due to short follow-up. Ten patients (90.9%) were male, and all had unilateral involvement, whereas the only female patient (9.1%) had bilateral disease at presentation. At admission, the mean age was 14.82 ± 17.27 years (range 2-60 years). Seven of 12 eyes (58.33%) had stage 3a1, three eyes (25%) had stage 3a2, and two eyes (16.67%) had stage 2b disease. The mean follow-up duration was 65.73 ± 41.81 months (range 18-131 months). At the last visit, visual acuity remained stable in six of 12 eyes (50%), deteriorated in two eyes (16.67%), and improved at least one Snellen line in four eyes (33.33%). Transient IOP elevation was noted in eight eyes (66.67%). Cataract progression was documented in seven (58.33%) eyes, and four had cataract surgery (33.33%). Cyberknife therapy and subsequent vitrectomy surgery were performed in one eye (8.33%) due to a secondary vasoproliferative tumor occurrence. No other dexamethasone implant-related complication was noted.
Conclusions: Intravitreal dexamethasone implant can be a viable adjunctive option, especially in youngsters, to avoid frequent general anesthesia and in adults having massive macular exudation in addition to the conventional ablation treatments in Coats' patients.
Trial registration: Retrospectively registered. The study adhered to the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee (Date: 12.02.2025, Number: 2025/05-24).
Keywords: Coats’ disease; Cyberknife; Dexamethasone intravitreal implant; Laser photocoagulation; Retinal telangiectasis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Dokuz Eylul University Training and Research Hospital (Approval No: 2025/05–24, Date: 12.02.2025). This study was retrospective in nature and based solely on pre-existing medical records. As such, no direct contact with patients occurred. In accordance with the decision of the ethics committee, informed consent was not required for this type of retrospective chart review. Competing interests: The authors declare no competing interests.
Figures


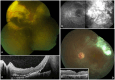

References
-
- Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol. 2001;131(5):561–71. - PubMed
-
- Tekin NF, Saatci AO, Kavukçu S. Vascular tortuosity and Coats’-like retinal changes in facioscapulohumeral muscular dystrophy. Ophthalmic Surg Lasers. 2000;31(1):82–3. - PubMed
-
- Tsai ASH, Wang CT, Lee TC, Nagiel A, Matsunaga K, Harper CA 3rd, et al. Clinical characteristics and treatment outcomes in unilateral coats disease: a global collaborative study. Ophthalmol Retina. 2025;9(6):570–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

