Recent Advancements in Metal-Organic Framework-Based Microfluidic Chips for Biomedical Applications
- PMID: 40731645
- PMCID: PMC12298669
- DOI: 10.3390/mi16070736
Recent Advancements in Metal-Organic Framework-Based Microfluidic Chips for Biomedical Applications
Abstract
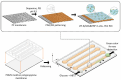
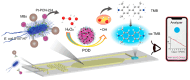
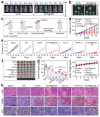
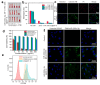
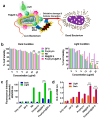
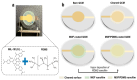
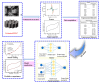
The integration of metal-organic frameworks (MOFs) with microfluidic technologies has opened new frontiers in biomedical diagnostics and therapeutics. Microfluidic chips offer precise fluid control, low reagent use, and high-throughput capabilities features further enhanced by MOFs' ample surface area, adjustable porosity, and catalytic activity. Together, they form powerful lab-on-a-chip platforms for sensitive biosensing, drug delivery, tissue engineering, and microbial detection. This review highlights recent advances in MOF-based microfluidic systems, focusing on material innovations, fabrication methods, and diagnostic applications. Particular emphasis is placed on MOF nanozymes, which enhance biochemical reactions for multiplexed testing and rapid pathogen identification. Challenges such as stability, biocompatibility, and manufacturing scalability are addressed, along with emerging trends like responsive MOFs, AI-assisted design, and clinical translation strategies. By bridging MOF chemistry and microfluidic engineering, these systems hold great promise for next-generation biomedical technologies.
Keywords: MOF-based nanozymes; biomedical application; cell migration; microfabrication; microfluidic chips; point-of-care diagnostics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Toxicity Challenges and Current Advancement in Metal-Organic Frameworks (MOFs) for Biomedical Applications.Biol Trace Elem Res. 2025 Jun 24. doi: 10.1007/s12011-025-04712-z. Online ahead of print. Biol Trace Elem Res. 2025. PMID: 40553239 Review.
-
Recent Advances in Metal-Organic Framework-Based Nanozymes for Intelligent Microbial Biosensing: A Comprehensive Review of Biomedical and Environmental Applications.Biosensors (Basel). 2025 Jul 7;15(7):437. doi: 10.3390/bios15070437. Biosensors (Basel). 2025. PMID: 40710087 Free PMC article. Review.
-
The fusion of metal-organic framework (MOF) and covalent organic framework (COF): A synergistic leap toward bridging boundaries in catalytic, sensing, and biomedical frontiers.Adv Colloid Interface Sci. 2025 Jul 22;344:103613. doi: 10.1016/j.cis.2025.103613. Online ahead of print. Adv Colloid Interface Sci. 2025. PMID: 40714298 Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Opportunities in functionalized metal-organic frameworks (MOFs) with open metal sites for optical biosensor application.Adv Colloid Interface Sci. 2025 Jul 7;344:103598. doi: 10.1016/j.cis.2025.103598. Online ahead of print. Adv Colloid Interface Sci. 2025. PMID: 40651338 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

