Pathologic Response and Survival Outcomes on HER2-Low vs. HER2-Zero in Breast Cancer Receiving Neoadjuvant Chemotherapy
- PMID: 40731799
- PMCID: PMC12298401
- DOI: 10.3390/medicina61071168
Pathologic Response and Survival Outcomes on HER2-Low vs. HER2-Zero in Breast Cancer Receiving Neoadjuvant Chemotherapy
Abstract
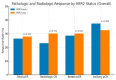
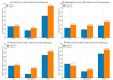
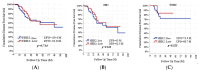
Background and Objectives: The clinical value of HER2-low breast cancer (BC), defined by immunohistochemistry (IHC) scores of 1+ or 2+/ISH-negative without HER2 amplification, remains unclear in the neoadjuvant setting. This study aimed to determine whether HER2-low and HER2-zero tumors differ in pathological complete response (pCR) rates and disease-free survival (DFS) among early-stage breast cancer patients undergoing neoadjuvant chemotherapy (NAC). Materials and Methods: We retrospectively analyzed 134 early BC patients treated with NAC between 2017 and 2023. Patients were categorized as HER2-zero (IHC 0) or HER2-low (IHC 1+ or 2+/ISH-). The primary endpoint was total pCR (tpCR); secondary endpoints included breast (bpCR), nodal (npCR), and radiologic complete response (rCR), alongside DFS analysis stratified by hormone receptor (HR) status. Results: Of the cohort, 91 patients (67.9%) were HER2-zero and 43 (32.1%) were HER2-low. There was no statistically significant difference in tpCR (26.4% vs. 27.9%, p = 0.852), bpCR (28.6% vs. 30.2%, p = 0.843), npCR (37.4% vs. 32.6%, p = 0.588), and rCR (23.1% vs. 30.2%, p = 0.374) between HER2-zero and HER2-low groups. DFS did not significantly differ between HER2-zero and HER2-low groups overall (p = 0.714), nor within HR-positive (p = 0.540) or TNBC (p = 0.523) subgroups. Conclusions: HER2-low tumors demonstrated similar pathological responses and survival outcomes compared to HER2-zero tumors. While a HER2-low status does not appear to define a distinct biological subtype in early BC, it remains a relevant classification for emerging HER2-targeted therapies, needing further investigation in prospective studies.
Keywords: HER2-low; breast cancer; disease-free survival; hormone receptor status; neoadjuvant chemotherapy; pathologic complete response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Pathologic response rates in HER2-low versus HER2-zero early breast cancer patients receiving neoadjuvant therapy: a systematic review and meta-analysis.Breast Cancer Res. 2025 Mar 15;27(1):39. doi: 10.1186/s13058-025-01989-9. Breast Cancer Res. 2025. PMID: 40089780 Free PMC article.
-
Effect of Estrogen Receptor on the Relationship Between HER2 Immunohistochemistry Score and Pathological Complete Response to Neoadjuvant Treatment in HER2-Positive Breast Cancer.Breast J. 2024 Oct 10;2024:8851703. doi: 10.1155/2024/8851703. eCollection 2024. Breast J. 2024. PMID: 39742361 Free PMC article.
-
Comprehensive analysis of HER2 Low Breast Cancer Response to Neoadjuvant Chemotherapy, a Retrospective Cohort Study.Clin Breast Cancer. 2025 Aug;25(6):e801-e817.e3. doi: 10.1016/j.clbc.2025.03.013. Epub 2025 Mar 25. Clin Breast Cancer. 2025. PMID: 40254501
-
Comparative efficacy and safety of pyrotinib plus trastuzumab versus trastuzumab plus pertuzumab and trastuzumab monotherapy in neoadjuvant treatment of HER2-positive breast cancer: A systematic review and meta-analysis.Cancer Treat Rev. 2025 Mar;134:102901. doi: 10.1016/j.ctrv.2025.102901. Epub 2025 Feb 17. Cancer Treat Rev. 2025. PMID: 39986012
-
Comparison of HER2-zero and HER2-low in terms of clinicopathological factors and survival in early-stage breast cancer: A systematic review and meta-analysis.Cancer Treat Rev. 2023 Apr;115:102538. doi: 10.1016/j.ctrv.2023.102538. Epub 2023 Mar 6. Cancer Treat Rev. 2023. PMID: 36898351
References
-
- Schettini F., Pascual T., Conte B., Chic N., Brasó-Maristany F., Galván P., Martínez O., Adamo B., Vidal M., Muñoz M., et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2020;84:101965. doi: 10.1016/j.ctrv.2020.101965. - DOI - PMC - PubMed
-
- Denkert C., Seither F., Schneeweiss A., Link T., Blohmer J.U., Just M., Wimberger P., Forberger A., Tesch H., Jackisch C., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22:1151–1161. doi: 10.1016/S1470-2045(21)00301-6. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous